NIOS Class 10 Science Chapter 7 Chemical Bonding solutions in Hindi and English Medium updated for new academic session. Students of National Institute of Open Schooling can learn here about Science chapter 7 explanation step by step in simple format.
Understanding Chemical Bonding
In the exploration of the natural world, one of the most fundamental concepts is chemical bonding. It’s the invisible force that holds atoms together, allowing them to combine in myriad ways to form the substances that constitute everything around us, from the air we breathe to the materials that build our world. This discussion delves into what chemical bonds are, the types of chemical bonds, and their profound impact on the properties of substances.
The Essence of Chemical Bonding
Chemical bonding arises from the need of atoms to achieve stability. At the heart of this stability lies the noble gas configuration—a state where an atom has a complete outer shell of electrons. Atoms, inherently seeking this stability, engage in chemical bonding. The result is the formation of molecules and compounds, whether through the combination of identical atoms, leading to molecules of elements, or through the union of different atoms, yielding compounds.
Types of Chemical Bonds
Chemical bonds can be broadly categorized into ionic and covalent bonds:
Ionic Bonding: This type of bond forms through the transfer of electrons from one atom to another, typically between a metal and a non-metal. The process results in the creation of charged particles or ions, where the loss of an electron forms a positively charged cation, while the gain of an electron creates a negatively charged anion. These ions then attract each other through electrostatic forces, forming an ionic bond. Sodium chloride (table salt) is a classic example of an ionic compound, where sodium donates an electron to chlorine, resulting in a stable lattice of oppositely charged ions.
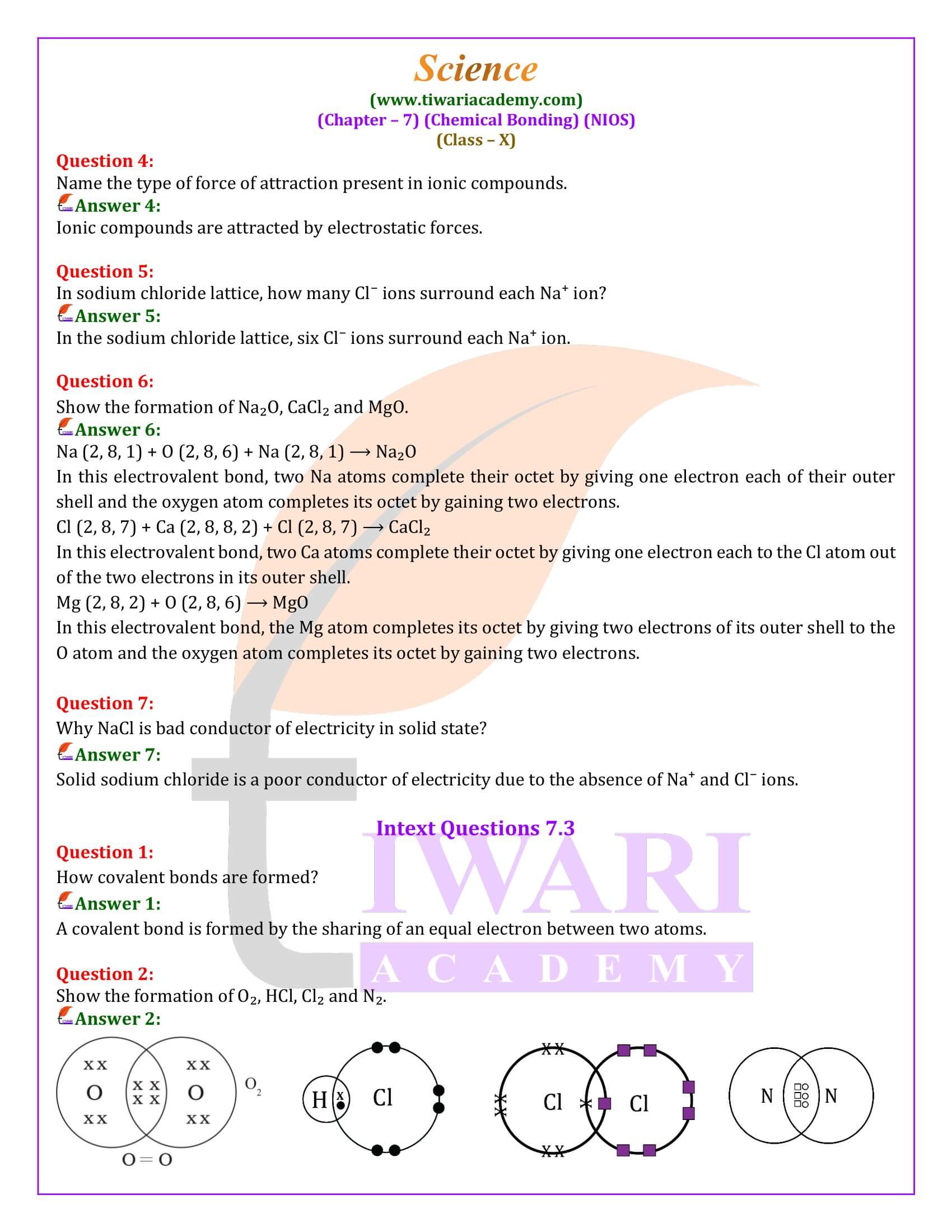
Covalent Bonding: Unlike ionic bonds, covalent bonds form through the sharing of electrons between atoms. This sharing allows each atom to achieve a stable electron configuration. Covalent bonds are common between non-metal atoms. The strength and number of these bonds can vary, leading to single, double, or triple bonds, each providing different stability and properties to the molecule. Water (H2O), oxygen (O2), and nitrogen (N2) are prime examples of covalent compounds.
Properties Influenced by Bond Type
The nature of the chemical bond significantly influences the properties of the resulting substance:
Ionic Compounds: Characterized by high melting and boiling points, these compounds are generally solid at room temperature. They are typically soluble in water but insoluble in organic solvents. Ionic compounds conduct electricity when molten or dissolved in water, due to the mobility of ions.
Covalent Compounds: These compounds can exist as gases, liquids, or solids at room temperature, often with lower melting and boiling points compared to ionic compounds. Covalent compounds do not conduct electricity, as they do not have free ions or electrons. They are usually soluble in organic solvents but insoluble in water.
The Impact of Chemical Bonding
Understanding chemical bonding is crucial for grasping the formation and properties of the vast array of substances we encounter. It explains why certain substances dissolve in water while others do not, why some materials conduct electricity, and why others are insulators. Beyond these practical implications, the study of chemical bonding is essential for the development of new materials, medicines, and technologies, making it a cornerstone of scientific advancement.
Chemical bonding not only explains the diversity and behavior of matter but also underpins the continuous innovation in science and industry. By mastering this fundamental concept, we unlock the potential to manipulate matter at its most basic level, paving the way for endless possibilities in research and application.