NIOS Class 10 Science Chapter 6 Periodic Classification of Elements all question answers and solutions in English and Hindi Medium. Get here National Institute of Open Schooling Science book chapter 6 notes and solution with complete explanation.
NIOS Class 10 Science Chapter 6 Periodic Classification of Elements
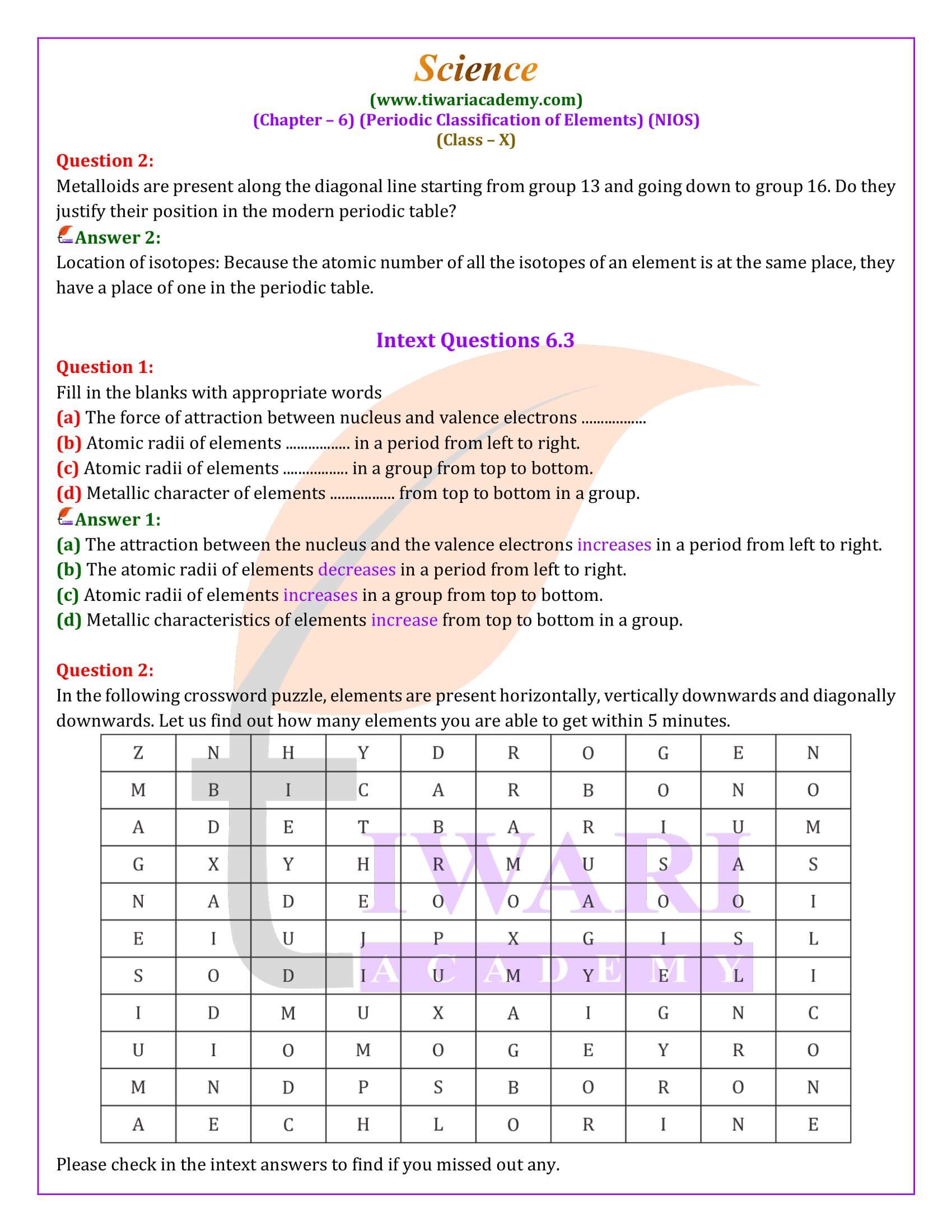
The Periodic Classification of Elements
The systematic arrangement of elements to study their properties and behavior has always been a cornerstone of chemistry. The journey of classifying elements, from early attempts to the modern periodic table, outlines the evolution of scientific thought and the quest for order in the natural world.
Early Attempts at Classification
Before the 18th century, only a handful of elements were known, making it relatively easy to study and remember their properties individually. However, by the mid-19th century, the discovery of over sixty elements and their numerous compounds made it imperative to find a systematic way to classify them. This necessity gave birth to various classification attempts, driven by the observation that elements share similarities among themselves. The first notable classification divided elements into metals and non-metals, though it was too simplistic and couldn’t accommodate elements like germanium and antimony that showed properties of both categories.
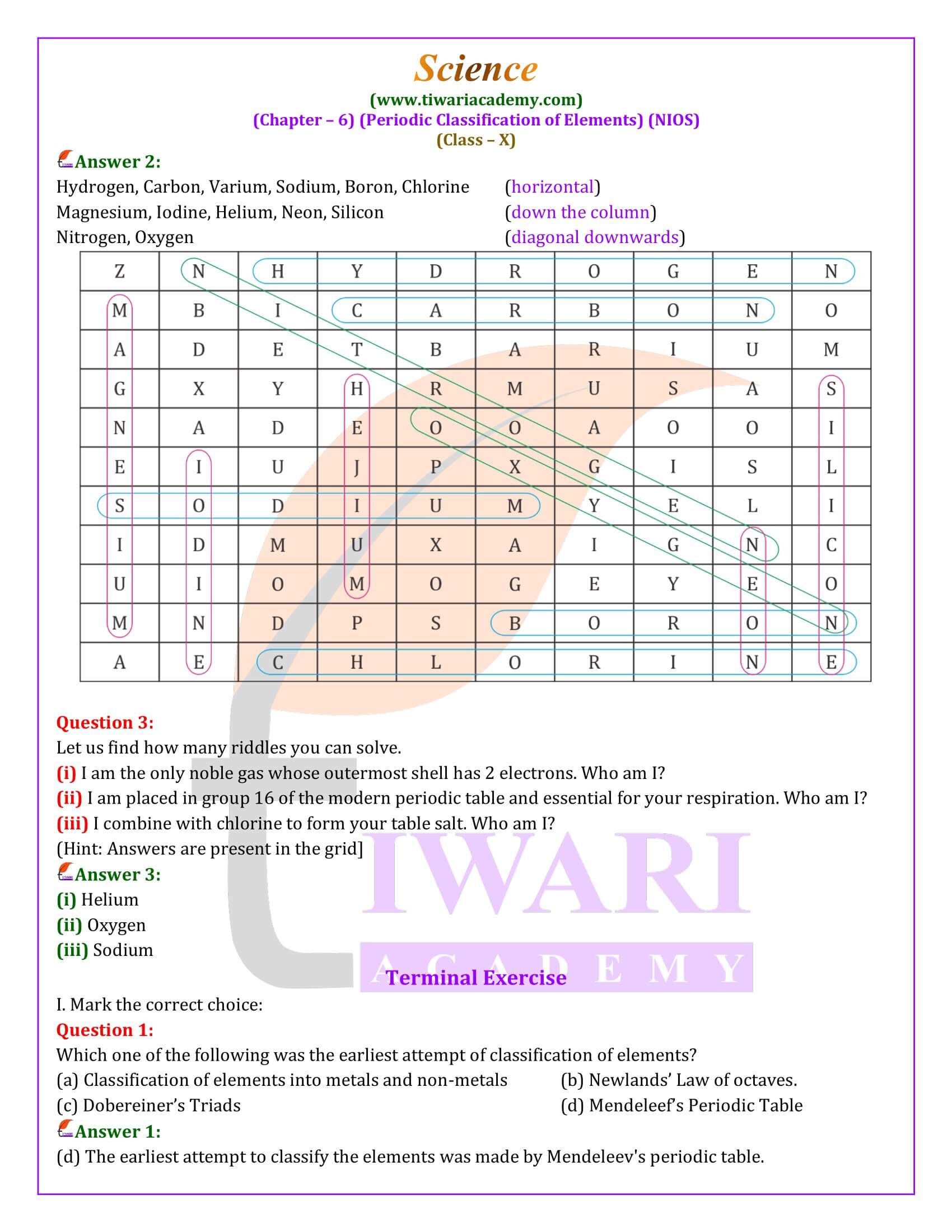
Breakthroughs in Periodic Classification
The quest for a reliable basis for classification led to the identification of atomic mass as a constant property of elements, paving the way for more sophisticated classification systems. Among the early significant attempts were Dobereiner’s Triads, Newlands’ Law of Octaves, Mendeleev’s Periodic Law and Table, and the Modern Periodic Table. Dobereiner’s Triads grouped elements into sets of three (triads) based on their atomic masses and chemical properties, though it applied to only a few elements. Newlands’ Law of Octaves, likening the pattern to musical scales, observed that every eighth element shared similar properties but was limited to elements with atomic masses less than 40 u.
Mendeleev’s Pioneering Work
Dmitri Mendeleev’s periodic law, which stated that the properties of elements are a periodic function of their atomic masses, marked a significant leap forward. Mendeleev arranged elements in a table based on their atomic masses and left gaps for yet-to-be-discovered elements, predicting their properties with remarkable accuracy. Although Mendeleev’s table included all known elements and addressed some of the classification challenges, it also had defects, such as the ambiguous positioning of hydrogen and the lack of provision for isotopes.
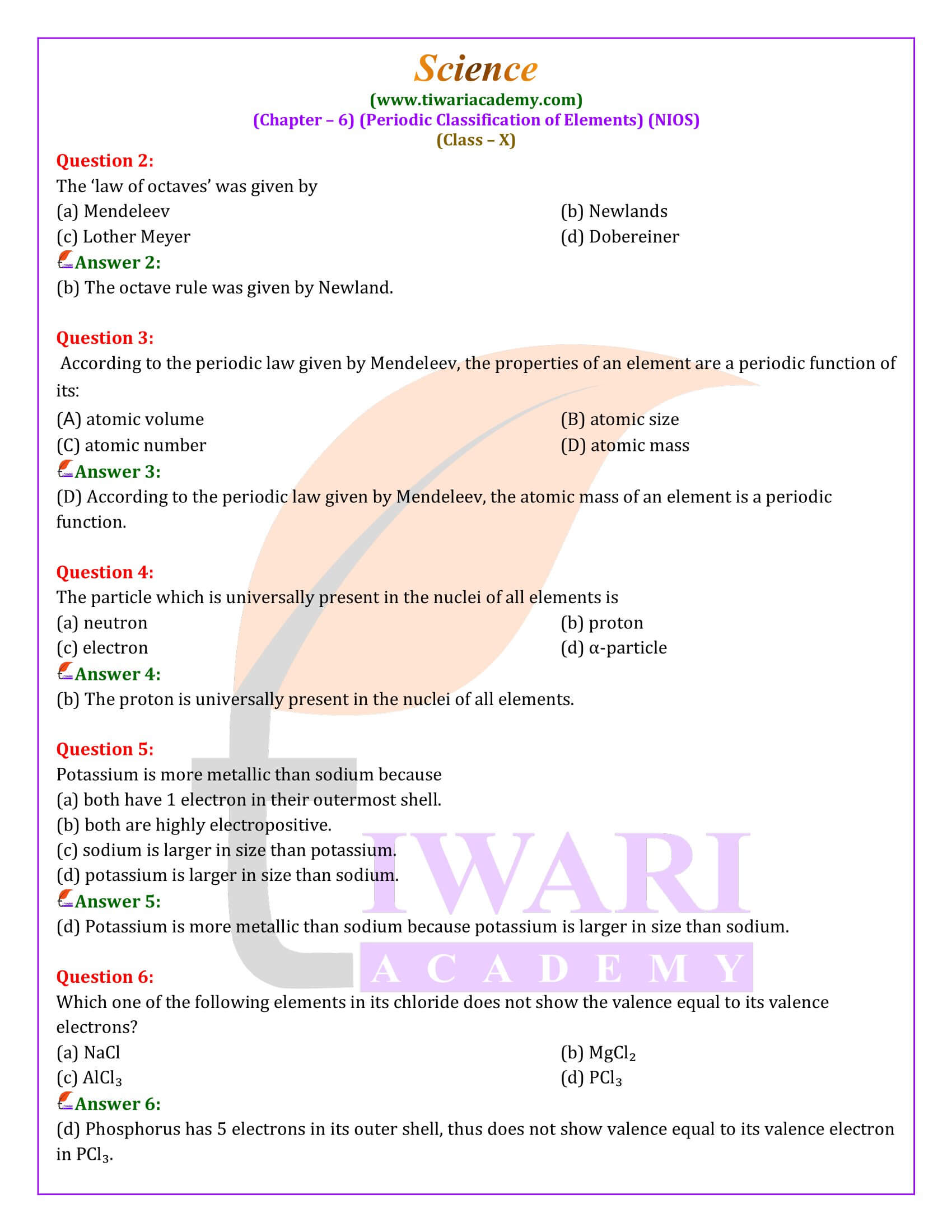
The Modern Periodic Law and Table
The discovery that the atomic number is a more fundamental property than atomic mass led to the Modern Periodic Law. This law states that the chemical and physical properties of elements are periodic functions of their atomic numbers. The Modern Periodic Table, arranged accordingly, overcomes the defects of Mendeleev’s table by accurately grouping elements, accommodating isotopes, and reflecting the periodicity of properties based on atomic structure and electron configuration.
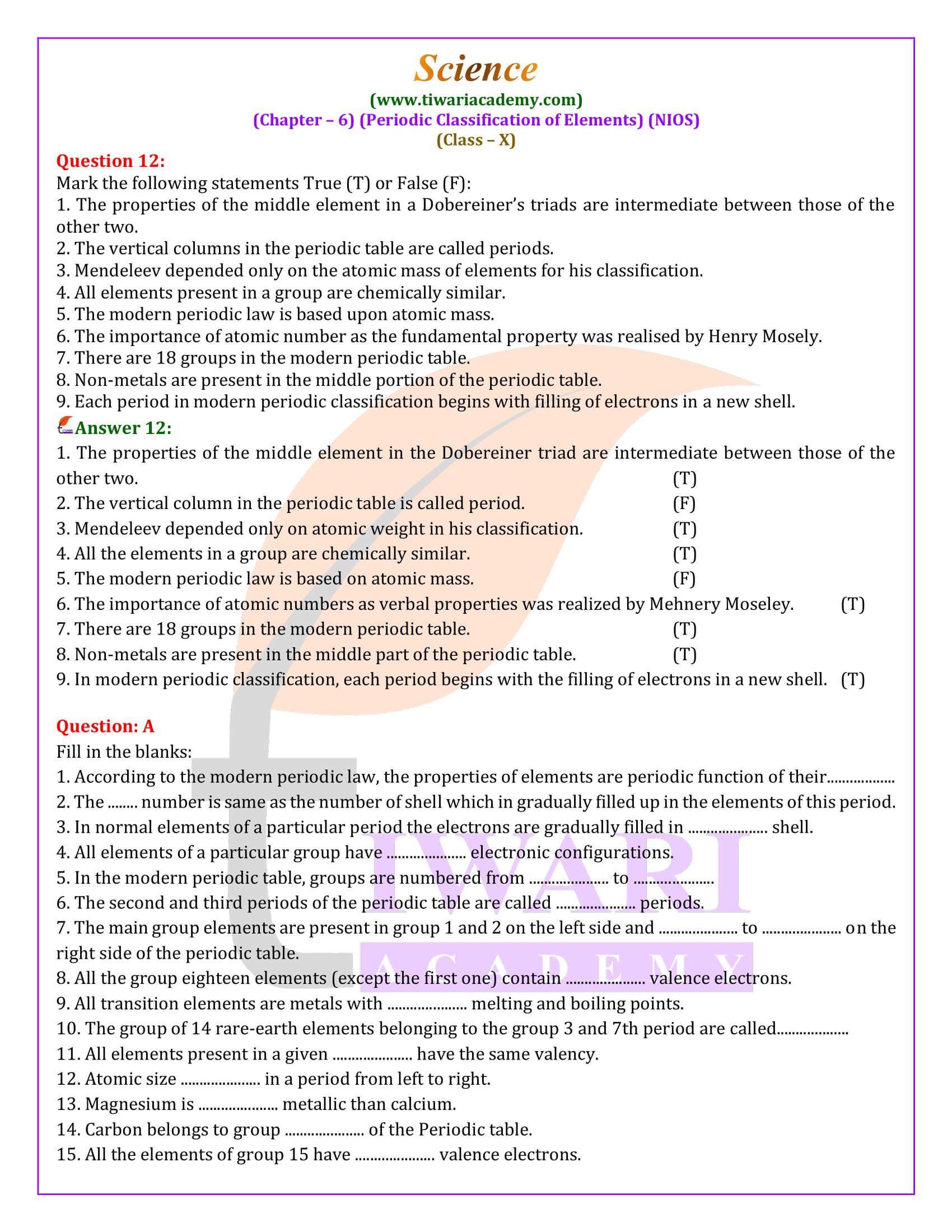
Periodic Trends in Properties
The Modern Periodic Table illustrates trends in atomic size, metallic character, and other properties across periods and groups. Atomic size decreases from left to right within a period and increases from top to bottom within a group. Similarly, metallic character exhibits a decrease across a period and an increase down a group, highlighting the underlying principles of electron configuration and atomic structure that govern the behavior of elements.
The classification of elements from ancient attempts to the modern periodic system showcases the evolution of our understanding of chemical elements. The periodic table, as it stands today, not only provides a comprehensive framework for studying elemental properties but also underscores the unifying principles of chemistry and the predictability of the natural world.