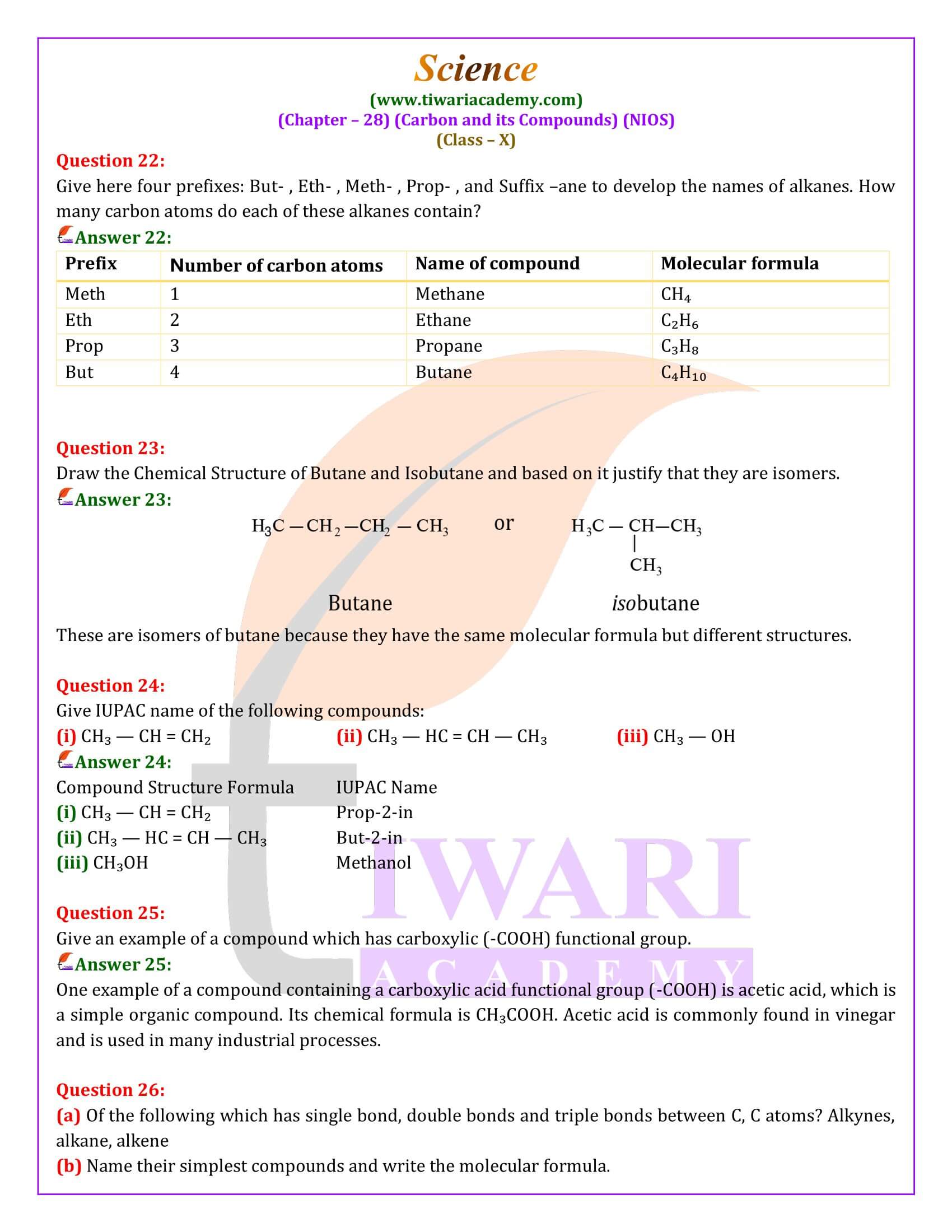
NIOS Class 10 Science Chapter 28 Carbon and its Compounds in Hindi and English Medium updated for academic session 2025-26. NIOS Class 10 Science Chapter 28 Question Answers is simplified in such a way that it makes learning this chapter very easy.
NIOS Class 10 Science Chapter 28 Carbon and its Compounds
Understanding Carbon and Its Compounds
Carbon, a fundamental element in the study of chemistry, demonstrates a vast array of chemical behaviors and forms numerous compounds, playing a crucial role in various natural and technological processes. As the sixth most abundant element in the universe, carbon is integral to life on Earth, contributing to the composition of all known life forms. Its ability to form a wide variety of compounds stems from its unique properties, such as the ability to form long chains and complex structures with itself and other elements.
Properties and Allotropes of Carbon
One of the key discussions in carbon chemistry is its allotropes—different forms in which carbon exists. NIOS Class 10 Science Chapter 28 discusses three primary allotropes: diamond, graphite, and fullerenes. Diamonds, formed under extreme conditions of high pressure and temperature inside the Earth, are renowned for their hardness and are used in cutting and drilling applications as well as in jewelry. Graphite, in contrast, is soft, conducts electricity, and is used in applications such as lubricants and electrodes. Fullerenes, discovered relatively recently, consist of carbon atoms arranged in a spherical structure and have potential applications in materials science due to their unique properties.
Hydrocarbons and Organic Chemistry
The vast domain of hydrocarbons—compounds consisting only of carbon and hydrogen—is an essential area of study. Hydrocarbons are categorized into alkanes, alkenes, and alkynes, based on the types of bonds between carbon atoms. This categorization helps in understanding their properties and reactions. Alkanes contain single bonds, alkenes include one or more double bonds, and alkynes feature triple bonds. These structural variations significantly influence their chemical reactivity and applications.
Functional Groups and Organic Reactions
Beyond simple hydrocarbons, carbon forms a wide range of compounds by substituting hydrogen atoms with functional groups like hydroxyl (-OH), carboxyl (-COOH), and others. These groups confer specific properties and reactivity patterns to the molecules they are part of, leading to a myriad of chemical behaviors. This adaptability makes carbon compounds central to biological processes and industrial applications, ranging from pharmaceuticals to plastics and beyond.
Everyday Compounds and Their Uses
NIOS Class 10 Science Chapter 28 covers several carbon-based compounds used daily, such as ethanol (found in alcoholic beverages and used as a solvent), acetic acid (the main component of vinegar), and various others like methanol and propanone (acetone). Understanding these compounds’ structure, production, and applications bridges the gap between theoretical chemistry and practical applications, illustrating the relevance of carbon chemistry in everyday life.
The Significance of Carbon Chemistry
The study of carbon and its compounds is vast and diverse, reflecting the element’s central role in both the environment and technology. From its structural versatility in forming both stable and complex molecules to its extensive use in industry and biology, carbon’s chemistry offers insights into both the natural world and human technological advancement. This exploration not only enriches our understanding of fundamental chemistry but also highlights the practical implications of carbon-based compounds in various scientific, environmental, and economic contexts.