NIOS Class 10 Science Chapter 2 Matter in Our Surroundings Question answers with explanation in Hindi and English Medium. Students of NIOS take the benefits of learning Science chapter 2.
NIOS Class 10 Science Chapter 2 Matter in Our Surroundings
Exploration of Matter in Our Surroundings
The study of matter, a substance that occupies space and has mass, forms the foundation for understanding our world. This exploration delves into the particulate nature of matter, distinguishing between its three states—solid, liquid, and gas—while explaining the effects of pressure and temperature on these states. Through examples and scientific principles, we grasp the inter-conversion among these states, their classification into elements, compounds, and mixtures, and the detailed nature of solutions, suspensions, and the methods used for separating mixtures.
Understanding Matter
Matter, encompassing everything that has mass and occupies space, is composed of tiny particles that clump together, yet are invisible to the naked eye. This includes all solids, liquids, and gases around us, from everyday objects like books and cars to natural elements like water and air. The study traces back to ancient philosophies and the advent of the atomic theory, highlighting the debate on matter’s divisibility and the eventual consensus on its particulate nature, defined by atoms and molecules.
States of Matter
Matter exists primarily in three states: solid, liquid, and gas. These states are differentiated by their properties—solids have fixed shapes and volumes, liquids have fixed volumes but take the shape of their containers, and gases neither have fixed shapes nor volumes. The transformation between these states is influenced by temperature and pressure, governed by the interplay between intermolecular forces and thermal energy.
Composition of Matter
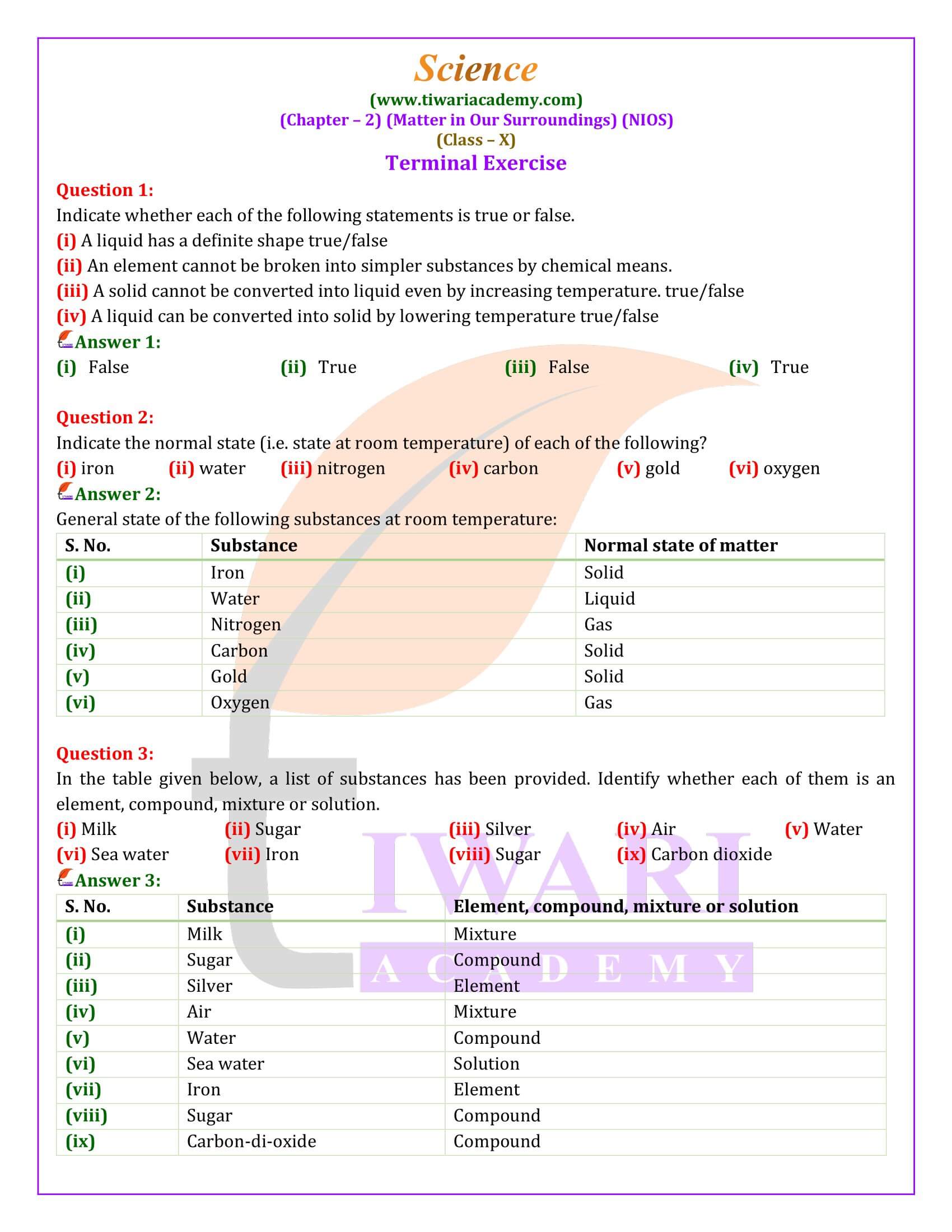
Matter is categorized into elements, compounds, and mixtures based on its composition. Elements are pure substances consisting of a single type of atom. Compounds result from the chemical combination of two or more elements in fixed proportions, possessing properties distinct from their constituent elements. Mixtures are physical combinations of two or more substances, classified as homogeneous (solutions) or heterogeneous (suspensions and colloids), each retaining its original properties.
Solutions and Suspensions
Solutions, homogeneous mixtures, are formed when solutes dissolve in solvents, exemplified by sugar or salt in water. The concentration of solutions is a crucial concept, indicating the amount of solute per unit of solvent. Suspensions, on the other hand, are heterogeneous mixtures where the solute particles are large enough to settle out over time, unlike the smaller particles in colloids.
Separating Mixtures
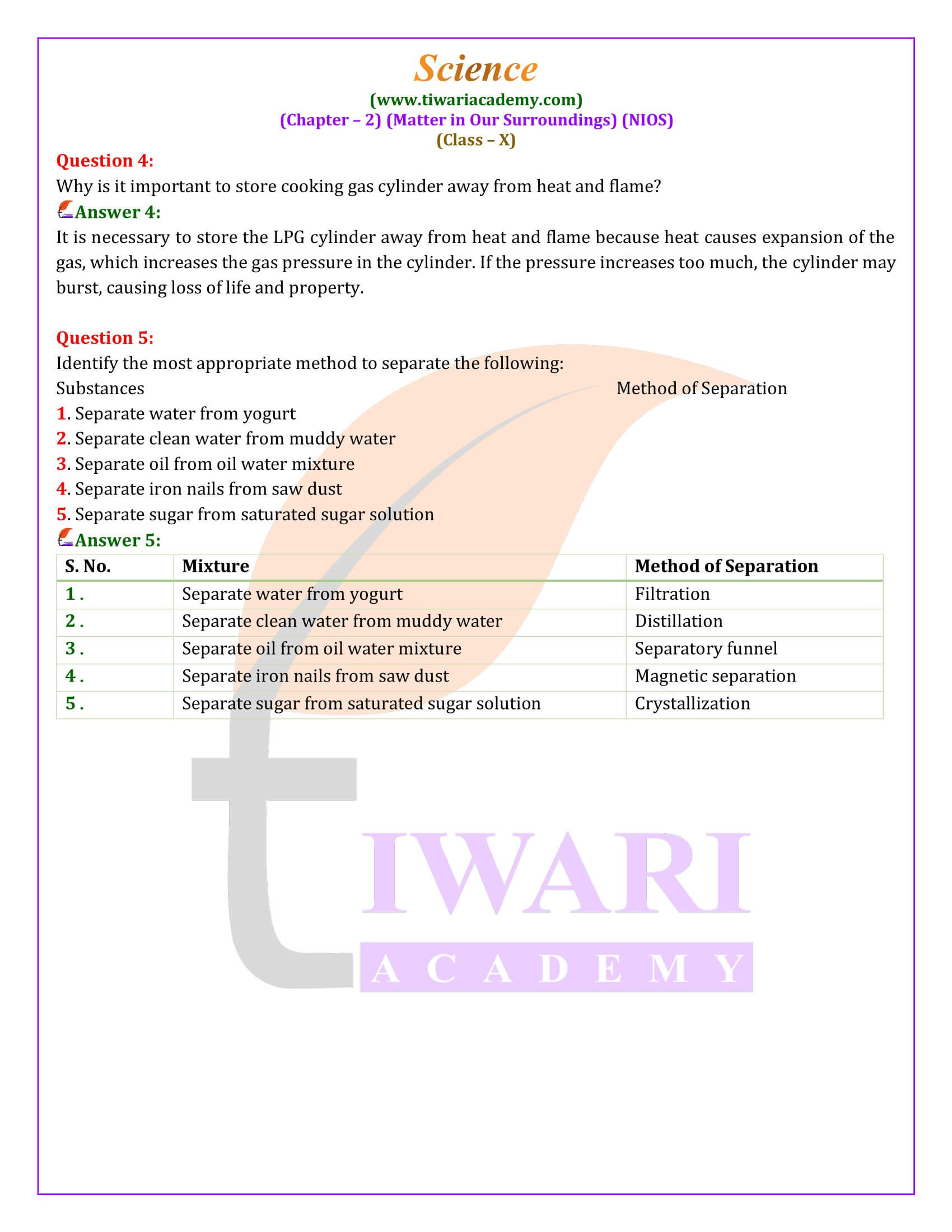
The separation of mixtures into their component substances is achieved through various physical methods based on differences in physical properties. Techniques such as filtration, distillation, crystallization, and magnetic separation are employed, each suitable for specific types of mixtures and desired components.
The exploration of matter in our surroundings reveals the intricate balance between physical and chemical properties that define the state and composition of matter. From the atomic and molecular understanding of elements and compounds to the practical aspects of solutions, suspensions, and the separation of mixtures, this journey highlights the foundational principles of matter that govern the natural and technological world around us.