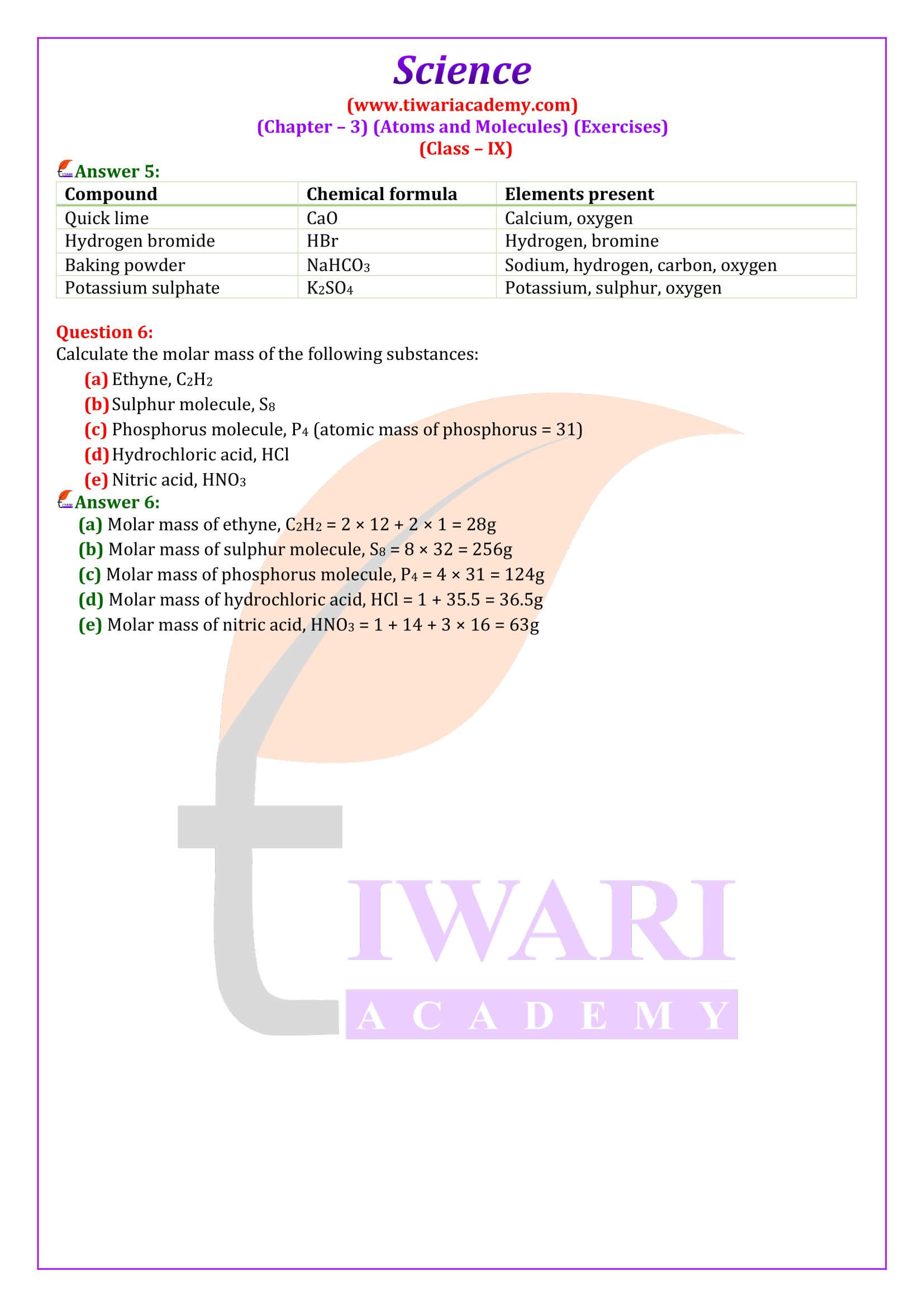
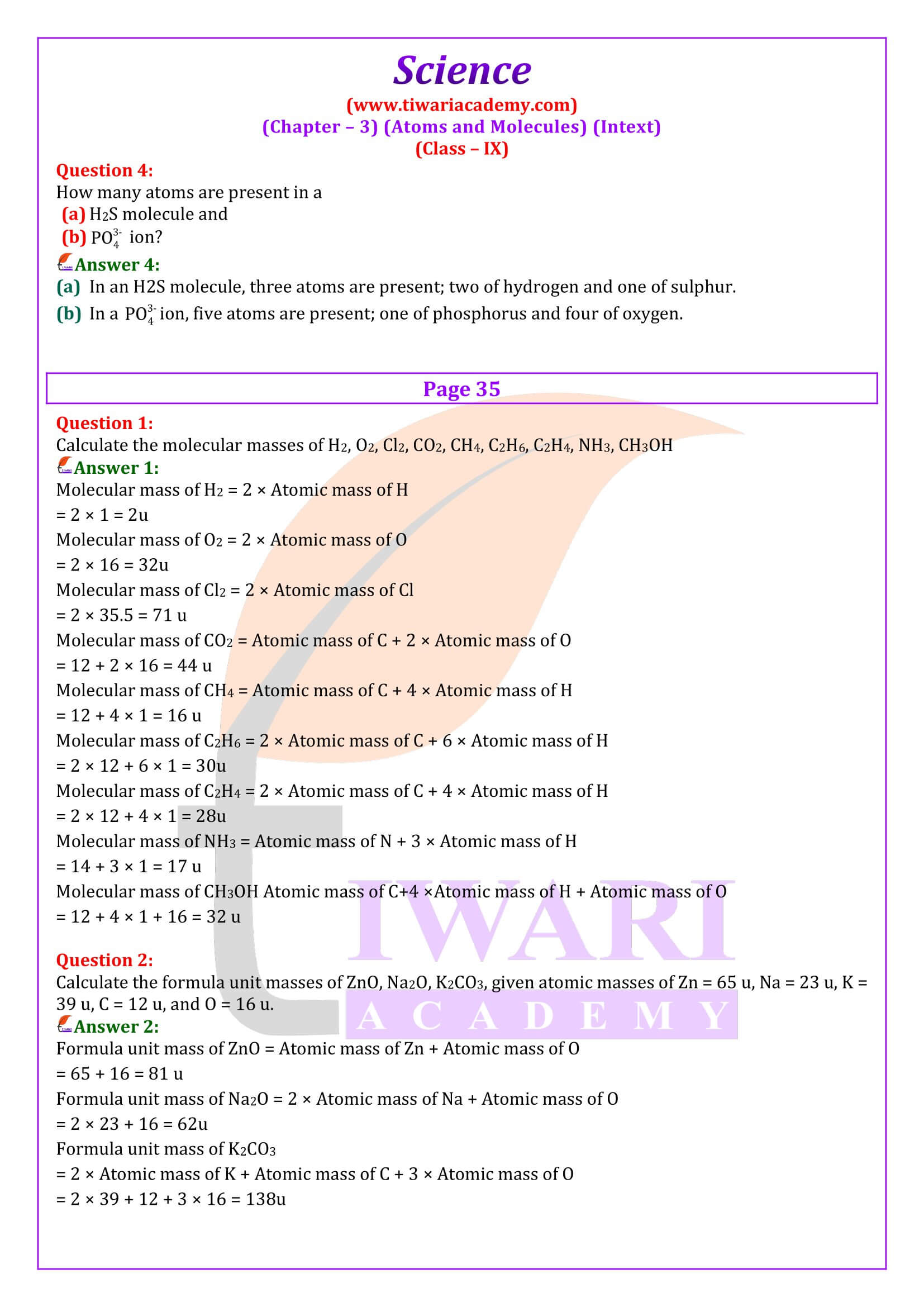
NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules download in PDF file updated for academic session 2025-26 in Hindi and English Medium based on rationalised NCERT Books published for 2025-26 examination.
NCERT Solutions for Class 9 Science Chapter 3
Class 9 Science Chapter 3 Question Answers
- Class 9 Science Chapter 3 Exercises
- Class 9 Science Chapter 3 Intext Questions
- Class 9 Science Chapter 3 Extra Questions
- Class 9 Science Chapter 3 Hindi Medium
- Class 9 Science Chapter 3 Notes in English
- Class 9 Science Chapter 3 Notes in Hindi
- Class 9 Science Chapter 3 NCERT Book
- Class 9 Science NCERT Solutions
- Class 9 all Subjects NCERT Solutions
The NCERT Class 9 Science textbook, Chapter 3 Atoms and Molecules introduces students to the world of atoms and molecules and covers various topics related to the structure and properties of these fundamental units of matter. The main topics covered in Chapter 3 are Laws of Chemical Combination that is Law of Conservation of Mass Law of Constant Proportions, What Are Atoms and Molecules? Definition of atoms and molecules Difference between atoms and molecules.
Chemical Formulae, Molecular Mass and Mole Concept
Class 9 Science chapter 3 describes the rules for writing chemical formulae of compounds Examples of chemical formulae, definition of molecular mass calculating the molecular mass of a compound Concept of the mole and Avogadro’s number. It helps in calculating the mass of one mole of a substance calculating the number of moles of a substance. Further we will study about Chemical Reactions and Chemical Equations, Balancing chemical equations, and Types of chemical reactions like combination, decomposition, displacement, double displacement.

UP Board High School students can download UP Board Solutions in Hindi Medium here. 9th Science chapter 3 Page 32, Page 33, Page 35, Page 39, Page 40, Page 42 and Exercises answers with explanation in English Medium or Page 36 ke Uttar, Page 40 ke Uttar, Page 44 ke Uttar, Page 46 ke Uttar, Page 48 ke Uttar and Abhyaas ke Uttar in Hindi Medium free to study online or in PDF form to use offline.
Atoms and Molecules in Everyday Life
Class 9 science chapter 3 explains about molecules in various substances (e.g., water, oxygen, carbon dioxide) Role of atoms and molecules in chemical reactions and definition of atomic mass Relative atomic masses of elements. Here we will learn about molecules of elements and compounds that is explanation of diatomic and polyatomic molecules and examples of diatomic molecules (e.g., hydrogen, oxygen, nitrogen). Mole Concept and Molar Mass are the considered as most important topics of the chapter. Learn here about relationship between the mole concept and molar mass, calculation of molar mass of compounds. Definitions of empirical and molecular formulae.

Download NCERT Solutions 2025-26 and NCERT Solutions Apps for other subjects are also in same format. All the contents are updated for new session based on latest CBSE Syllabus.
A recap of the main concepts and ideas presented in the chapter 3 Atoms and Molecules, serves as an introduction to the fundamental concepts of chemistry, including the composition of matter at the atomic and molecular level. It provides the groundwork for further exploration of chemical reactions, stoichiometry, and the structure of atoms and molecules in later chapters of the textbook.

Preparing for NCERT Class 9 Science Chapter 3 requires a strategic approach to understanding the fundamental concepts of chemistry. For this purpose, read the Chapter Thoroughly. Begin by reading the chapter carefully from the NCERT textbook. Pay attention to definitions, examples, and key points. While reading, take detailed notes on important concepts, definitions, formulas, and key terms. Summarize the main ideas of each section. The Law of Conservation of Mass and the Law of Constant Proportions and their significance.
| Class: 9 | Science |
| Contents: | NCERT Solutions and Important Questions |
| Chapter 3: | Atoms and Molecules |
| Content Type: | PDF, Text and Videos |
| Academic Year: | 2025-26 |
| Medium: | English and Hindi Medium |
Focus on learning how to write chemical formulae of compounds correctly. Practice writing formulae for various compounds. Understand the concept of molecular mass and its calculation. Practice calculating the molecular mass of compounds. Learn the mole concept and Avogadro’s number. Understand how to calculate the number of moles in a given mass of a substance. Practice balancing chemical equations of different types of reactions, including combination, decomposition, displacement, and double displacement.

Atomic Mass and Atomic Molecules
Learn the concept of atomic mass and understand the relative atomic masses of elements. Understand the distinction between diatomic and polyatomic molecules, and recognize examples of diatomic molecules. Learn the relationship between the mole concept and molar mass. Practice calculating the molar mass of compounds. Understand how to determine empirical and molecular formulae for compounds.
9th Science Chapter 3 Answers in English & Hindi Medium

NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules Exercise and Intext questions of all pages are given below to free download in PDF form. All the contents can be downloaded without any login or password.
Chemical Reactions in Everyday Life
Recognize the presence of molecules and atoms in everyday substances and chemical reactions. Understand their role in various processes. Practice solving numerical problems related to molecular mass, moles, and chemical equations. Work through exercises and examples in the textbook. Utilize diagrams and visual aids to help understand atomic and molecular structures. Supplement your learning with online resources, videos, and interactive simulations related to atoms and molecules. These can provide additional clarity. Group study can offer different perspectives and help reinforce your understanding. Regularly revise your notes and the key points of the chapter. Repetition is key to retaining information.

Tiwari Academy offers explanations and videos that break down complex concepts in the chapter 3 of 9th science. Visual aids and clear explanations can help students grasp difficult topics more easily. The online website provides additional practice questions and exercises related to Chapter 3. Practicing these questions can help students reinforce their understanding of the chapter and improve their problem-solving skills.
Concise and well-structured revision notes specific to Chapter 3 of 9th Science available on Tiwari Academy. These notes can help students review key concepts and important points quickly before exams. Some online platforms, including Tiwari Academy, offer online tests and mock exams that simulate the real exam environment. This is valuable for self-assessment and exam preparation.

We have updated all the contents for new session 2025-26 for CBSE, UP Board, MP Board, etc., who are following NCERT Books and latest CBSE Syllabus.
Tiwari Academy provide access to previous years’ question papers and solutions. Solving these papers can help students become familiar with the exam pattern and practice time management. Tiwari Academy platforms have discussion forums or communities where students can interact, share their experiences, and seek advice from peers.
Extra Questions on 9th Science Chapter 3
Why is a cation so named?
When electric current is passed through the solution of a salt like sodium chloride (NaCl), the positive ion (Na+) migrates towards cathode (negative electrode). It is therefore, called cation. Remember this:
(i) Positive ion migrating towards cathode on passing electric current is known as cation.
(ii) Negative ion migrating towards anode on passing electric current is known as anion.
The atomic mass of an element is in fraction. What does it means?
If the atomic mass of an element is in fraction, this means that it is existed in the form of isotopes. The atomic mass is the average atomic mass and it is generally fractional.
Why are chemical reaction according to law of conservation of mass?
In all chemical reactions, there is only exchange of reactants taking place when products are formed. Since there is no loss or gain of mass, the chemical reactions are according to law of conservation of mass.
Give one limitation of law of constant composition.
The law of constant composition does not hold when different isotopes of an element take part in the formation of a particular compound. For example,
In carbon dioxide (CO2)
By using C-12 isotopes, the ratio of C : O is 12 : 32.
By using C-14 isotopes, the ratio of C : O is 14 : 32.
This means that the two ratios are not the same.
Out of atoms and molecules which can exist independently?
Molecules can exist independently. However, the atoms of noble gases (He, Ne, Ar, Kr, Xe) can also exist independently.
Questions for Practice on 9th Science Chapter 3

Question 1
In what respect does Dalton’s Atomic theory hold good even today?
Answer 1:
atom has been found to be made up of sub-atomic particles like electrons, protons and neutrons. But it is still the smallest particle of matter which can take can take part in chemical combination.

Question 2:
Why is it necessary to balance a chemical equation?
Answer 2:
A chemical equation has to be balanced in order to satisfy the law of conservation of mass. According to the law, there is no change in mass when the reaction changes into the production. Therefore, the chemical equation has to be balanced.

Question 3:
What is basic different between atoms and molecules?
Answer 3:
Atoms except those of noble or inert gas elements cannot exist of their own. However, all molecules can have independent existence.

From an examination perspective, NCERT Class 9 Science Chapter 3 Atoms and Molecules, is important for several reasons. Chapter 3 introduces students to fundamental concepts in chemistry, including atoms, molecules, chemical formulae, and chemical equations. These concepts are essential for building a strong foundation in chemistry, and they serve as the basis for more advanced chemistry topics in higher classes.

Questions related to Chapter 3 are frequently included in Class 9 Science examinations. This chapter is a crucial part of the curriculum, and students can expect to see questions about atoms, molecules, chemical equations, and related calculations in their exams. Class 9 science chapter 3 involves solving numerical problems related to calculating molecular masses, determining the number of moles, and balancing chemical equations. These problems test students’ ability to apply mathematical concepts to chemistry, which is a valuable skill for exams and further studies in science.
Understanding chemical reactions and balancing chemical equations is a fundamental aspect of chemistry. This chapter introduces students to different types of chemical reactions and provides a basis for studying chemical reactions in more detail in higher classes. Chapter 3 lays the groundwork for stoichiometry, which is the quantitative study of reactants and products in chemical reactions. A solid grasp of stoichiometry is crucial for solving more complex chemical problems in future chemistry courses.

The concepts covered in this chapter 3 of 9th science have real-world applications. For example, understanding the concept of moles and molecular masses is important in various fields, including chemistry, biology, and pharmacy. The knowledge gained from this chapter is revisited and expanded upon in later classes and chapters. A strong foundation in Chapter 3 is, therefore, essential for success in future science studies.
In summary, NCERT Class 9 Science Chapter 3 is important not only for scoring well in exams but also for building a solid foundation in chemistry. It introduces students to key concepts and problem-solving skills that are valuable for their academic and scientific development.
Practice Questions
Important Questions on 9th Science Chapter 3
When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combinations will govern your answer?
Carbon + Oxygen → Carbon dioxide 3g of carbon reacts with 8 g of oxygen to produce 11g of carbon dioxide. If 3g of carbon is burnt in 50g of oxygen, then 3g of carbon will react with 8 g of oxygen. The remaining 42 g of oxygen will be left un-reactive. In this case also, only 11g of carbon dioxide will be formed. The above answer is governed by the law of constant proportions.
What is the mass of 4 mole of aluminium atoms (Atomic mass of aluminium = 27)?
The mass of 4 moles of aluminium atoms is (4 × 27)g = 108g
Convert into mole: 12g of oxygen gas.
32 g of oxygen gas = 1 mole Then, 12g of oxygen gas = 12/32 mole = 0.375 mole
What is the mass of 0.2 mole of oxygen atoms?
Mass of one mole of oxygen atoms = 16g Then, mass of 0.2 mole of oxygen atoms = 0.2 × 16g = 3.2g