NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure in PDF file format English and Hindi Medium updated for new academic session 2025-26 based on rationalised NCERT Books published for 2025-26 exams.
NCERT Solutions for Class 9 Science Chapter 2
Class 9 Science Chapter 2 Question Answers
- Class 9 Science Chapter 2 Exercises
- Class 9 Science Chapter 2 Intext Questions
- Class 9 Science Chapter 2 MCQ
- Class 9 Science Chapter 2 Extra Questions
- Class 9 Science Chapter 2 Hindi Medium
- Class 9 Science Chapter 2 Notes in English
- Class 9 Science Chapter 2 Notes in Hindi
- Class 9 Science Chapter 2 NCERT Book
- Class 9 Science NCERT Solutions
- Class 9 all Subjects NCERT Solutions
In the NCERT (National Council of Educational Research and Training) Class 9 Science textbook, Chapter 2 Is Matter around Us Pure? explores various topics related to the purity of matter and the separation of mixtures. The main topics covered in Chapter 2 are definition of a pure substance and a mixture, difference between pure substances and mixtures, Types of Mixtures: Homogeneous mixtures (solutions) and Heterogeneous mixtures.
Solvent and solute expressing the concentration of solutions in terms of solute mass, volume of the solution, and mass by mass percentage. We have to learn the definitions of suspensions and colloids, Differences between suspensions, colloids, and true solutions. Class 9 Science chapter 2 explores various methods of separation of mixtures like Handpicking, Winnowing, Sieving, Magnetic separation, Sublimation, Separation by filtration, Separation by decantation, Separation by evaporation, Separation by centrifugation, Separation by chromatography, Separation by distillation.
UP Board students can download UP Board Solutions for class 9 Science chapter 2 in Hindi Medium. Class 9 Science Chapter 2 question-answers of Page 15, Page 18, Page 24 and Exercises in English Medium or Page 16 ke Uttar, Page 20 ke Uttar, Page 26 ke Uttar, Page 27 ke Uttar and Abhyaas ke Uttar in Hindi Medium. NCERT Solutions 2025-26 of other subjects are also available free to download in PDF file format. Download Class 9 Apps for offline use. Download NCERT Solutions Offline Apps based on latest NCERT Books 2025-26, which works offline without internet.
Physical and Chemical Changes
Differentiating between physical and chemical changes Examples of physical and chemical changes. In class 9 science chapter we learn about types of Pure Substances like Elements and compounds Characteristics of elements and compounds. Differentiating between mixtures, compounds, and elements. Law of Conservation of Mass – Statement of the law Illustration through examples.
A recap of the main concepts and ideas presented in chapter 2, Is Matter Around Us Pure?, focuses on helping students understand the composition of matter, the classification of mixtures, methods for separating mixtures, and the distinction between pure substances and mixtures. These concepts are fundamental to chemistry and provide the basis for further exploration of chemical reactions and properties of matter in subsequent chapters of the textbook.

| Class: 9 | Science |
| Contents: | English and Hindi Medium Solutions |
| Chapter 2: | Is Matter Around Us Pure |
| Academic Year: | Session 2025-26 |
| Medium: | English and Hindi Medium |
Preparing for NCERT Class 9 Science Chapter 2 requires a strategic approach to understanding the concepts related to the purity of matter and the separation of mixtures effectively. Here are some strategies to prepare for this chapter. Begin by reading the chapter carefully from the NCERT textbook. Pay attention to definitions, examples, and key points. While reading, make concise notes of important concepts, definitions, and the steps involved in various separation methods.
Understand the classification of matter into pure substances and mixtures. Learn the differences between homogeneous mixtures (solutions) and heterogeneous mixtures. Practice solving problems related to the concentration of solutions. Understand how to calculate mass by mass percentage, volume by volume percentage, and other concentration measures. Focus on the various separation methods mentioned in the chapter. Understand when and how to use methods like filtration, distillation, chromatography, and more.
9th Science Chapter 2 Answers in English & Hindi Medium
NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure Intext Questions on different pages and Exercises question answers are given below in updated format. All the answers are checked by experts for academic session 2025-26. Download Class 9 all Subjects App for free use.
If possible, conduct simple experiments to understand the separation techniques practically. This can help in visualizing the processes and reinforcing your learning. Solve the exercise questions given at the end of NCERT books and examples provided in the textbook. Practice solving problems related to separation methods and concentration calculations. Consult additional resources such as reference books, online tutorials, and videos to clarify any doubts or delve deeper into specific topics if needed.
Discuss concepts and solve problems with classmates. Group study can be helpful in understanding different perspectives and learning from one another. Regularly review your notes and the key points of the chapter to reinforce your understanding. Utilize online educational platforms like websites or apps like Tiwari Academy offline and online apps that offer supplementary materials, such as videos, quizzes, and interactive simulations related to the chapter. Solve previous year’s question papers to become familiar with the types of questions that may appear in exams.
Extra Questions on 9th Science Chapter 2
Colloidal solution show Tyndall effect but true solution do not. Discuss.
In a colloidal solution, the particle size is such (1 nn to 100 nm), that these particles scatter the light rays as they fall on them. Because of scattering the path of the light as well as the particle become visible. But in a true solution, the particle size is so small (less than 1 nm) that these particles are not in a position to scatter the light. Therefore, true solution does not show any Tyndall effect.
Why do not the dispersed phase particles in a colloidal solution combine with one other?
They do not come closer because of the presence of either positive or negative charge on them. Due to mutual repulsion, these particles remain scattered in a colloidal solution.
What is the function of fractionating column in fractional distillation?
A fractionating column obstructs the upwards movement of the vapours of the liquids. As a result, the energy (latent heat of fusion) which is released by the high boiling liquid is taken by the low boiling liquid. It remains in the vapours state. The high boiling liquid by releasing energy condenses and falls back in the distillation flask. Thus, fractionating column helps in the separation of the components from a mixture.
Alloys are sometimes called substitutional solid solutions. Explain.
Alloys are the homogeneous mixtures of two or more metals. For example, brass is a mixture of copper and zinc. Actually, copper is a crystalline solid in which the atoms are closely packed to form a crystal lattice. Some of these atoms have been replaced or substituted by atoms of zinc. Therefore, brass is regarded as a substitutional solid solution.
All mixtures are homogeneous. Is this statement correct? Justify your answer.
No, this statement is wrong. Mixture because they have combined with each other to form ammonium chloride which is a new substance. A mixture is always formed by mixing non-reacting substances.
Ammonia (gas) + Hydrogen chloride (gas) → Ammonium chloride (solid).
If you have any doubts or find certain topics challenging, don’t hesitate to seek help from your teacher or classmates. Clarify your doubts as soon as possible. Keep your notes, textbooks, and study materials organized for easy access during revision and exam preparation. Remember that Chapter 2 provides the foundational knowledge for understanding the composition of matter and various techniques used in chemistry. It is important to grasp these concepts thoroughly as they will be revisited and built upon in future science studies.
Questions for Practice on 9th Science Chapter 2
Tiwari Academy provides online support in all the subjects from class 1st to class 12. We can help students in preparation for NCERT Class 9 Science Chapter 2 in various ways. We offer detailed and step-by-step solutions to all the questions and exercises in the NCERT Class 9 Science textbook for Chapter 2. These solutions can help students understand how to approach and solve different types of problems related to the purity of matter and the separation of mixtures.
Concise and well-structured revision notes specific to Chapter 2 available on Tiwari Academy. These notes can help students review key concepts and important points quickly before exams. Some online platforms, including Tiwari Academy, provide online tests and mock exams that simulate the real exam environment. This is valuable for self-assessment and exam preparation. Tiwari Academy’s online resources are accessible 24/7, allowing students to study at their own pace and convenience.
This flexibility is particularly helpful for students who want to review the chapter multiple times. Online platforms offer options for students to submit their doubts or questions related to Chapter 2. Expert educators or tutors may respond to these doubts, providing clarification and assistance. Tiwari Academy provide access to previous years’ question papers and solutions. Solving these papers can help students become familiar with the exam pattern and practice time management. Students should explore the platform to determine which resources are most helpful for their individual learning needs and preferences.

Question 1:
A diamond knife is quite often used for cutting glass. Why?
Answer 1:
Diamond is probably the hardest substance known. Therefore, a knife made from a special type of diamond is used for cutting the glass.
Question 2:
Explain how does soap help in cleaning dirty clothes?
Answer 2:
In dirty clothes, the dust particles are present on oil are present on oil drops sticking to them. Simple water cannot remove these oil drops from the clothes because water and oil as such do not form a stable emulsion. Soap plays the role of emulsifier and helps in forming a stable emulsion between the two. This means that soap helps in removing these oil drops along with the dirt sticking to them. The dirty clothes get washed by soap solution.
From an examination point of view, NCERT Class 9 Science Chapter 2 holds significant importance for several reasons. Questions from Chapter 2 are often included in Class 9 Science examinations. This chapter is considered an integral part of the curriculum, and students can expect to see questions related to the purity of matter, types of mixtures, separation techniques, and concentration calculations in their exams.
Chapter 2 of 9th science delves into fundamental concepts related to the classification of matter into pure substances and mixtures. It also covers various methods of separating mixtures. A solid understanding of these concepts is crucial for scoring well in exams and for building a foundation in chemistry. Examinations include questions that require students to apply their knowledge of separation methods to real-world scenarios. This highlights the practical importance of the chapter’s content.
The chapter 2 of class 9 science includes numerical problems related to concentration calculations and other concepts. These problems test students’ ability to apply mathematical concepts to scientific situations, which is a valuable skill for exams. Understanding the separation techniques discussed in the chapter is not only useful for exams but also for practical applications in laboratory settings. Students may be asked to perform experiments related to these techniques during practical exams. Class 9 Science chapter 2 serves as a foundation for more advanced topics in chemistry that students will encounter in higher classes. A strong grasp of the concepts in this chapter is essential for success in future chemistry studies.
Question 3:
How will you justify that rusting of iron is a chemical change?
Answer 3:
The rust is a brown chemical compound known as hydrated ferric oxide. It cannot be removed from the surface of the metal b any means. Formula of a rust shows that iron has undergone a chemical change.
Important Questions on 9th Science Chapter 2
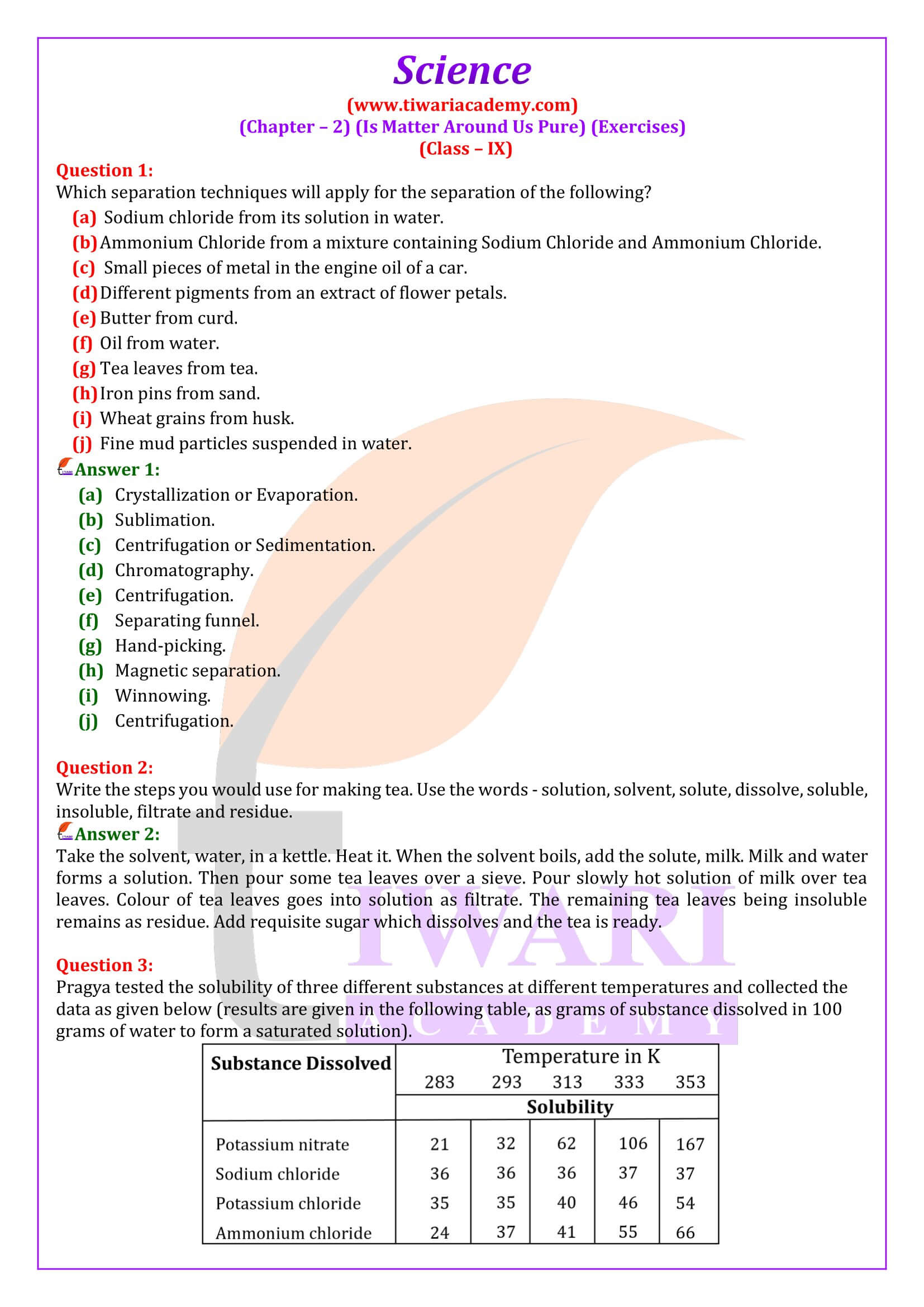
Write the steps you would use for making tea. Use the words – solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Take the solvent, water, in a kettle. Heat it. When the solvent boils, add the solute, milk. Milk and water forms a solution. Then pour some tea leaves over a sieve. Pour slowly hot solution of milk over tea leaves. Colour of tea leaves goes into solution as filtrate. The remaining tea leaves being insoluble remains as residue. Add requisite sugar which dissolves and the tea is ready.
What do you understand by a Saturated solution?
Saturated Solution: A solution in which no more of the solid (solute) can be dissolved at a given temperature is called a saturated solution. Suppose 50 gm of a solute is the maximum amount that can be dissolved in 100 gm water at 298 K. Then 150 gm of solution so obtained is the saturated solution at 298 K.
Describe Pure substance with example.
Pure Substance: A pure substance consists of a single of matter or particles and cannot be separated into other kind of matter by any physical process. Pure substances always have the same colour, taste and texture at a given temperature and pressure. For example, pure water is always colourless, odorless and tasteless and boils at 373 K at normal atmospheric pressure.
Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air, soil, vinegar, filtrated tea.
Homogeneous mixture – soda water, air, vinegar, filtered tea. Heterogeneous mixture – wood, soil.
How would you confirm that a colourless liquid given to you is pure water?
Every liquid has a characteristic boiling point at 1 atmospheric pressure. If the given colourless liquid boils exactly at 373 K at 1 atmospheric pressure, then it is pure water. If the boiling point is different, then the water is contaminated.
The knowledge gained from chapter 2, of class 9 science, will be revisited and expanded upon in higher classes, particularly in Class 10 and beyond. Building a strong foundation in this chapter is, therefore, crucial for future science studies. In summary, NCERT Class 9 Science Chapter 2 is important both for scoring well in exams and for building a strong foundation in chemistry. Students should dedicate time and effort to comprehensively learn the concepts presented in this chapter, as they are fundamental to the understanding of matter and its properties.