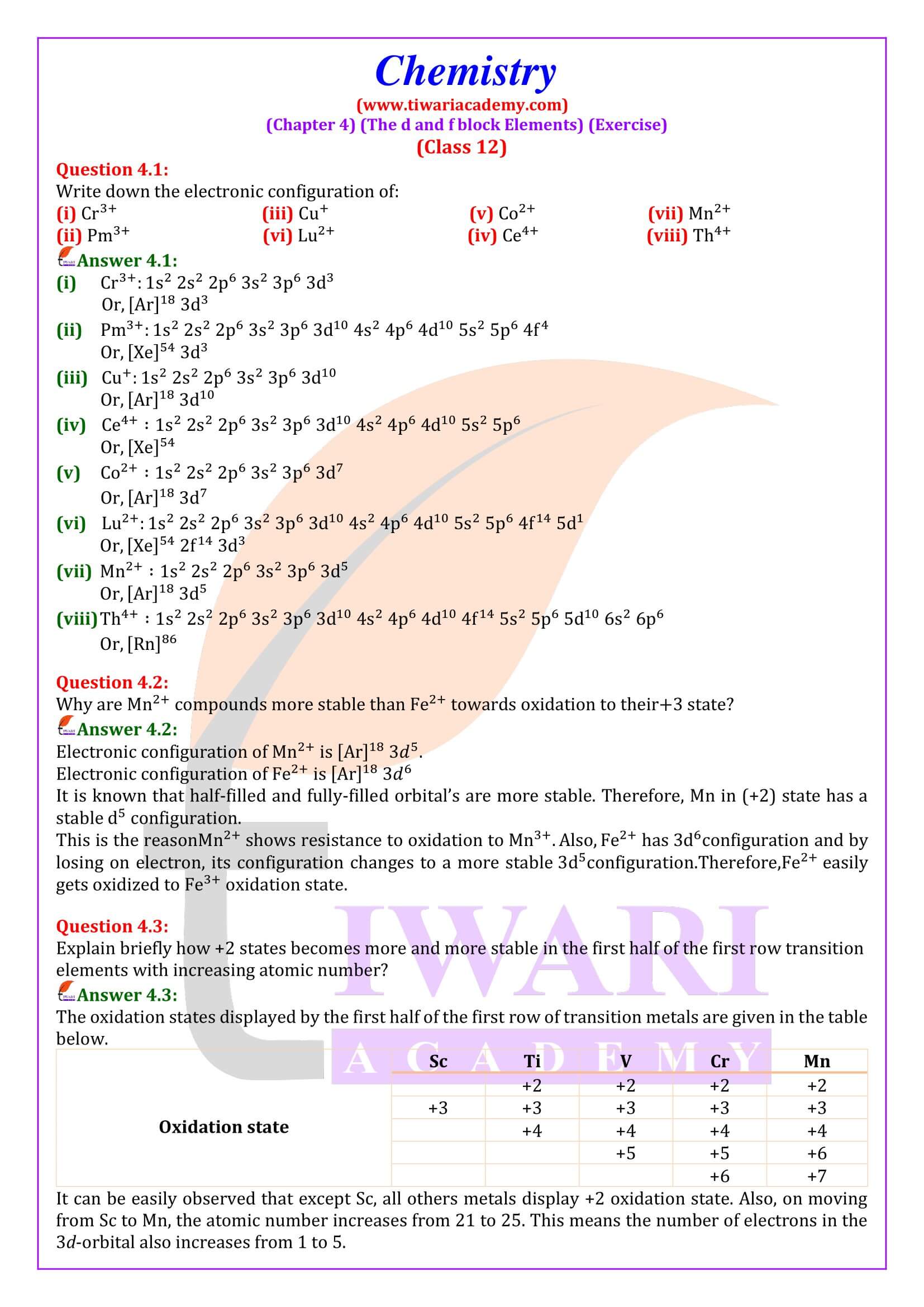
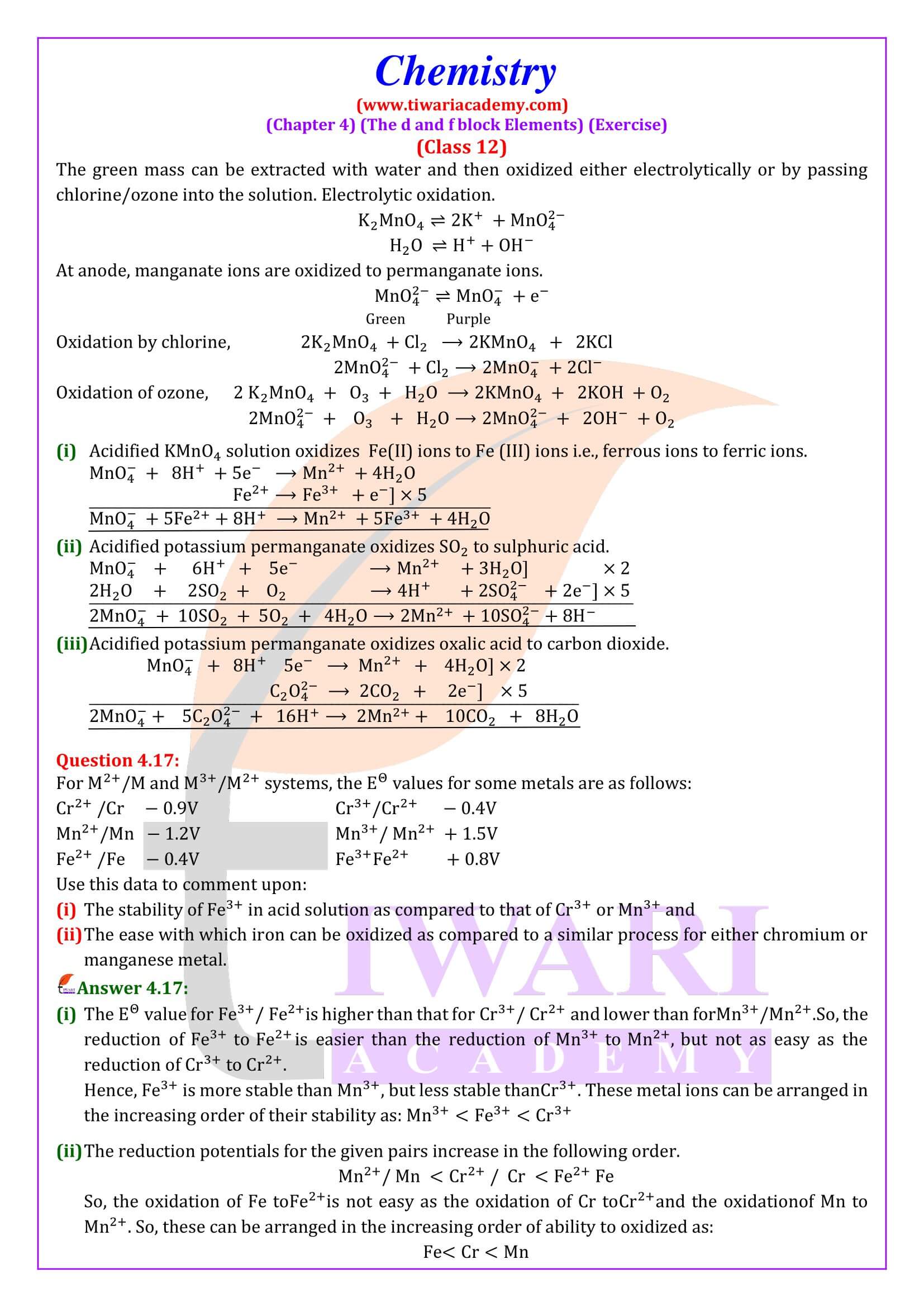
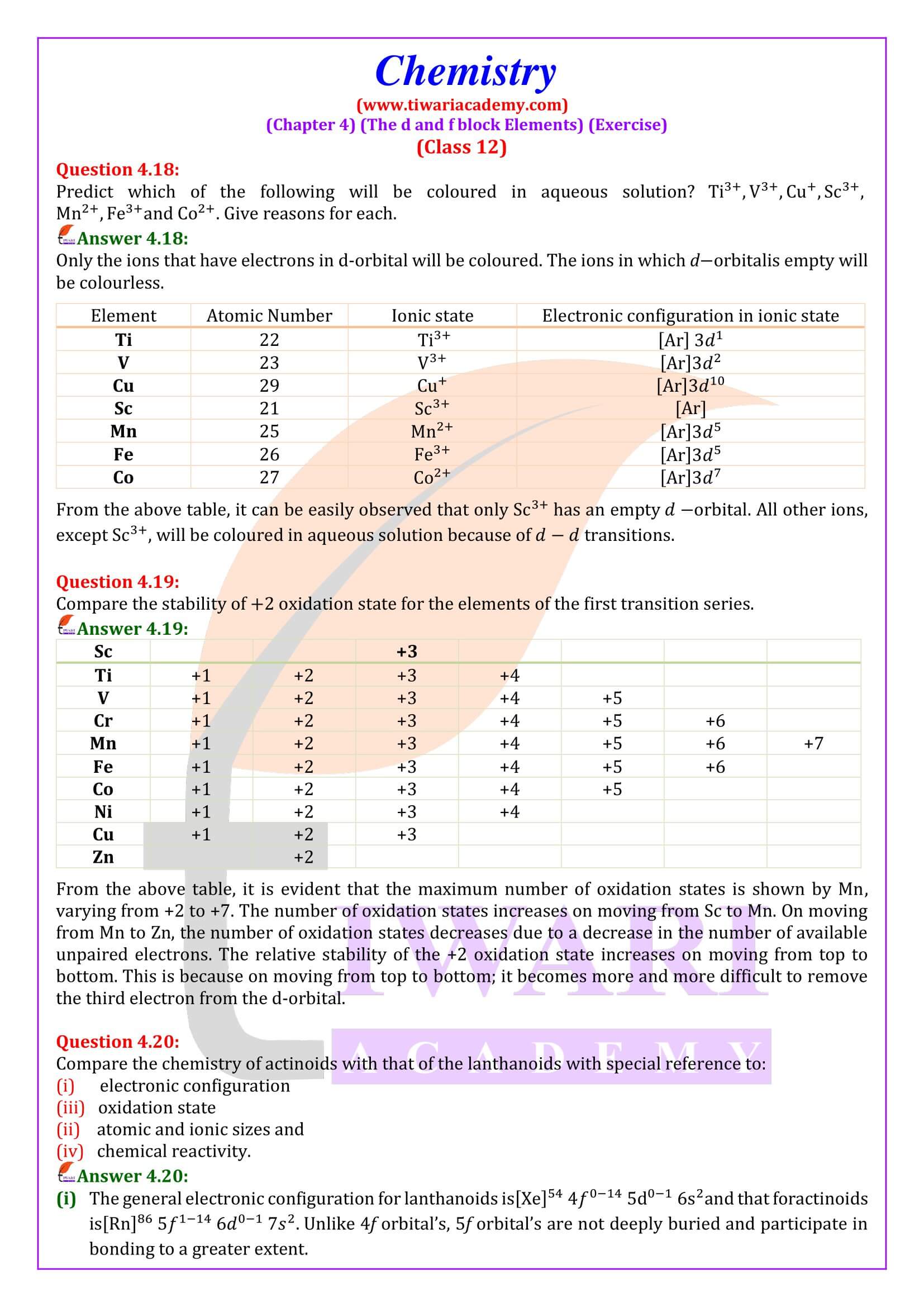
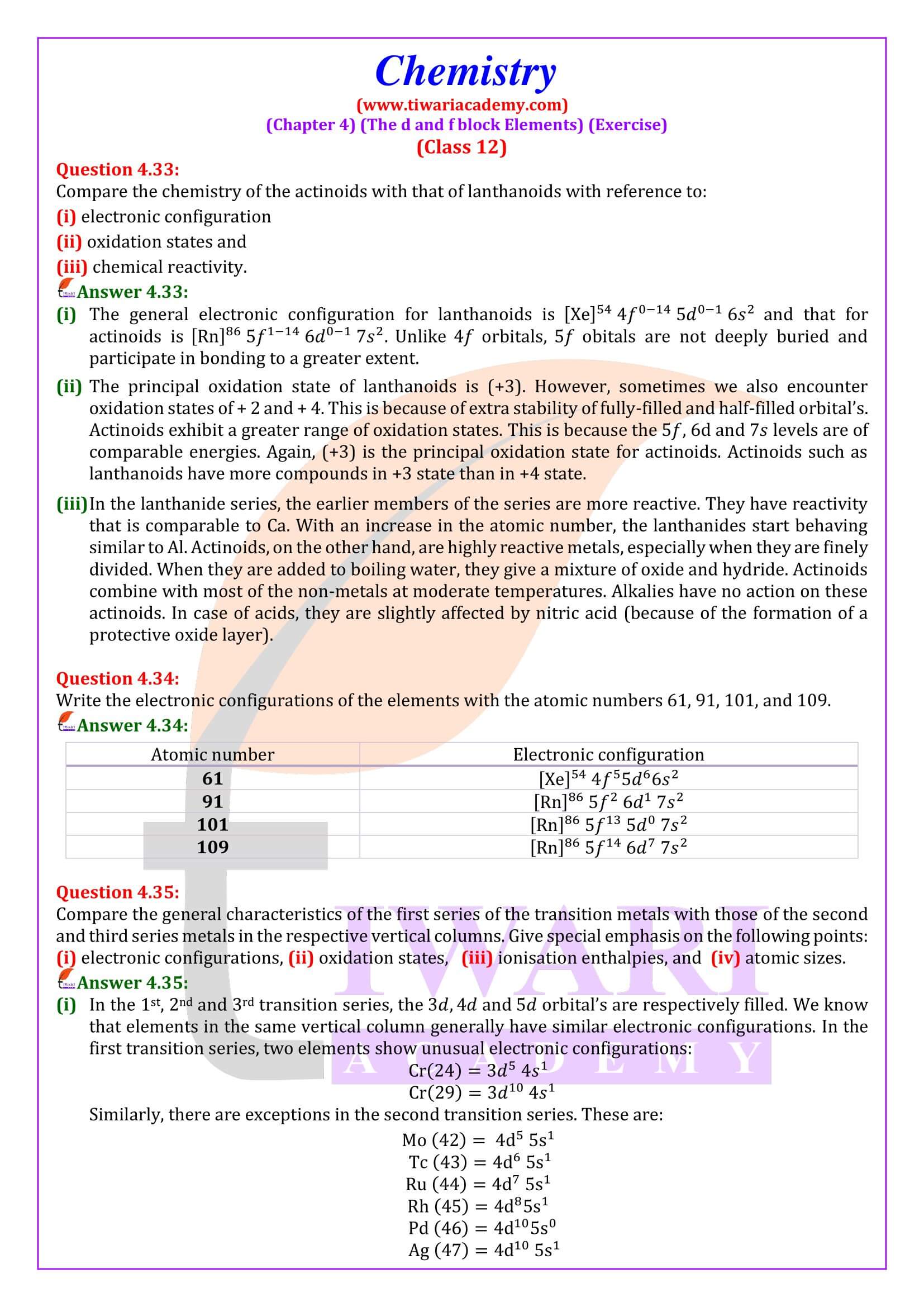
NCERT Solutions for Class 12 Chemistry Chapter 4 the d- and f- Block Elements in Hindi and English Medium updated for CBSE 2025-26 exams. Students of state board can also take the benefits during their study of intext and exercises questions in 12th Chemistry unit 4.
Practical Viva Question for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry Chapter 4
Class 12 Chemistry Chapter 4 the d and f Block Elements Question Answers
| Class: 12 | Chemistry |
| Chapter: 4 | The d and f Block Elements |
| Content: | Intext Exercises and NCERT Solutions |
| Mode of Study Material: | PDF, Images, Videos and Text |
| Session: | Academic Year 2025-26 |
| Medium: | Hindi and English Medium |
D block elements
D block elements are the elements that can be found from the third group to the twelfth group of the modern periodic table. They’re called “D-block elements” because they have their valence electrons in one or more d-orbitals (but not in any p-orbitals). Elements belong to d – group are
- 3d- Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn.
- 4d- Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd.
- 5d- La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg.
- 6d- incomplete.
The f block elements are the lanthanides and actinides and are called the inner transition elements because of their placement in the periodic table due to their electron configurations.
The elements of the f-block are (from atomic number 57 to 103 elements) as follows:
Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium etc.
Class 12 Chemistry Chapter 4 MCQ
Shape of d-orbital is
Which one of the following metals is used as a catalyst in the Haber’s process?
Which of the following is most stable in aqueous solution?
Transition Metals
Transition metals are defined as metals which have incomplete d sub-shell either in neutral atom or in their ions. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation of chemical bonds—in two shells instead of only one.
Examples: Various precious metals such as silver, gold and platinum and industrially important metals like iron, copper and titanium belong to the transition metals series.
Electronic Configurations of the d-Block Elements
In general, the electronic configuration of outer orbitals of these elements is
(n-1)d¹⁻¹⁰ ns¹⁻². The (n–1) stands for the inner d orbitals which may have one to ten electrons and the outermost ns orbital may have one or two electrons.
The Inner Transition Elements (f-BLOCK)
The f-block consists of the two series, lanthanoids (the fourteen elements following lanthanum) and actinoids (the fourteen elements following actinium). Because lanthanum closely resembles the lanthanoids, it is usually included in any discussion of the lanthanoids for which the general symbol Ln is often used. Similarly, a discussion of the actinoids includes actinium besides the fourteen elements constituting the series.
12th Chemistry Unit 4 Multiple Choice Questions
Which block of elements are known as transition elements?
Transition elements form alloys easily because they have
Which of the following is not an element of first transition series?
Electronic Configurations
It may be noted that atoms of these elements have electronic configuration with 6s² common but with variable occupancy of 4f level. However, the electronic configurations of all the tripositive ions (the most stable oxidation state of all the lanthanoids) are of the form 4f n (n = 1 to 14 with increasing atomic number).
General Characteristics
All the lanthanoids are silvery white soft metals and tarnish rapidly in air. The hardness increases with increasing atomic number, samarium being steel hard. Their melting points range between 1000⁰ to 1200⁰ K but samarium melts at 1623⁰ K. They have typical metallic structure and are good conductors of heat and electricity.
Density and other properties change smoothly except for Eu and Yb and occasionally for Sm and Tm. Many trivalent lanthanoid ions are coloured both in the solid state and in aqueous solutions. Colour of these ions may be attributed to the presence of f electrons.
Some Applications of d- and f-Block Elements
Iron and steels are the most important construction materials. Their production is based on the reduction of iron oxides, the removal of impurities and the addition of carbon and alloying metals such as Cr, Mn and Ni. Some compounds are manufactured for special purposes such as TiO for the pigment industry and MnO₂ for use in dry battery cells.
The battery industry also requires Zn and Ni/Cd. The elements of Group 11 are still worthy of being called the coinage metals, although Ag and Au are restricted to collection items and the contemporary UK copper coins are copper-coated steel. The silver UK coins are a Cu/Ni alloy. Many of the metals and/or their compounds are essential catalysts in the chemical industry.
Class 12 Chemistry Chapter 4 Important Questions
Why do the transition elements exhibit higher enthalpies of atomisation?
Because of large number of unpaired electrons in their atoms they have stronger interatomic interaction and hence stronger bonding between atoms resulting in higher enthalpies of atomisation.
What is the importance of f-block elements?
Uses of f-Block Elements
Some applications of the f-block elements are: Lanthanide alloys (mischmetal) utilized for the creation of instrumental steels and heat resistance. Carbides, Borides, and nitrides of lanthanides come in use as refractories. Lanthanide oxides come in use in cleaning glass as abrasives.
Why are f-block elements called rare earth metals?
Inner transition metals are those in which inner f-orbital is progressively filled, f-block elements are called inner transition metals. They are also called rare earth metals because they are rarely found in earth crust.