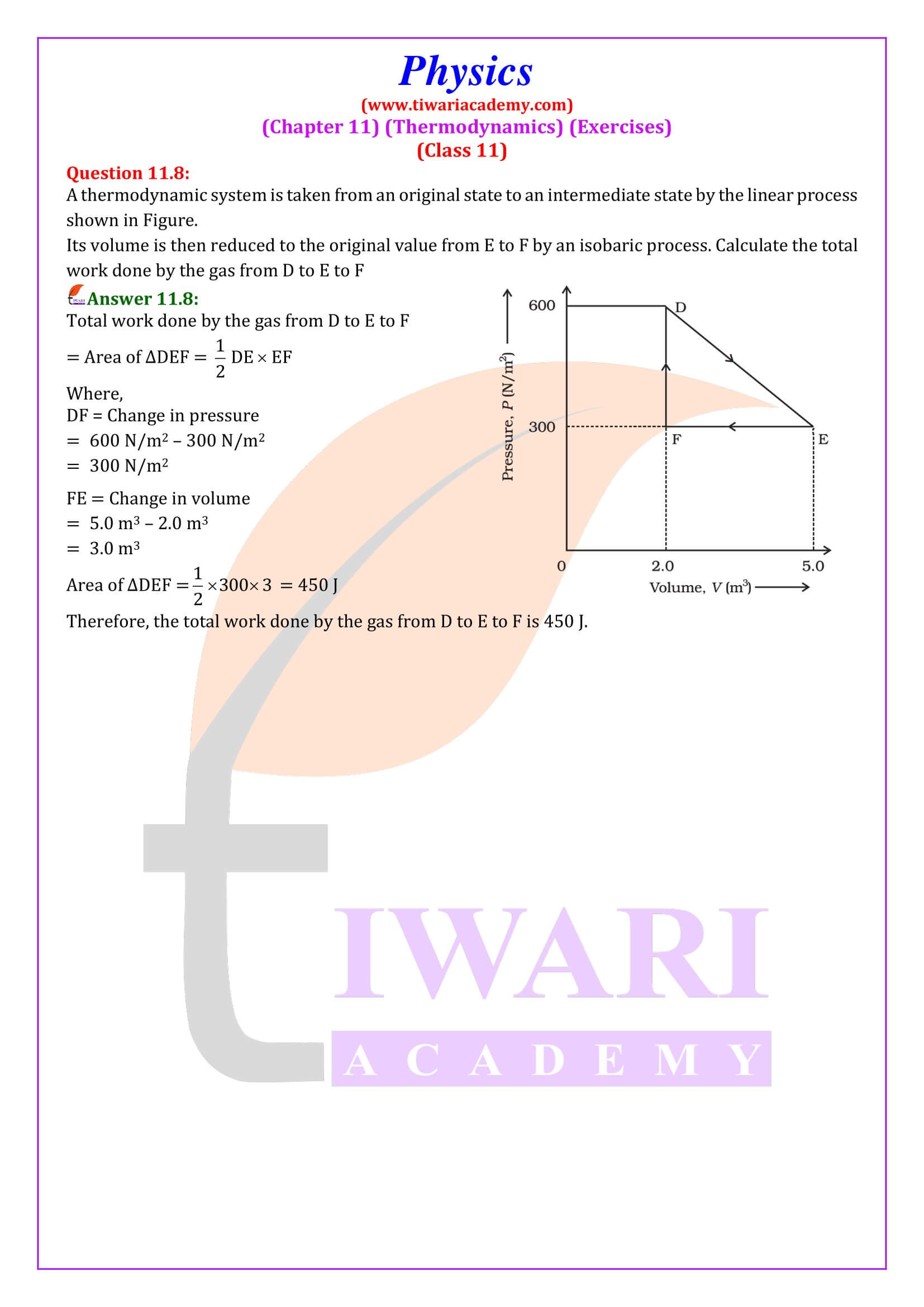
NCERT Solutions for Class 11 Physics Chapter 11 Thermodynamics in Hindi and English Medium updated for CBSE and State board academic session 2025-26 with MCQ and Extra Important Question answers. As you gain confidence in the important topics, start attempting mock tests and solving sample question papers. This will help you get accustomed to the exam pattern and time management.
Class 11 Physics Chapter 11 Thermodynamics Question Answers
Class 11 Physics Chapter 11 MCQ
Which of the following statement is correct for any thermodynamics system?
Heat cannot by itself flow from a body at lower temperature to a body at higher temperature is a statement as a consequence of
Which of the following parameters does not characterize the thermodynamic state of matter?
For a diatomic gas change in internal energy for a unit change in temperature for constant pressure and constant volume in u1 and u2 respectively. What is the ratio u1 and u2?
The First Law of Thermodynamics in Chapter 11 of 11th Physics
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but it cannot be created or destroyed. Internal energy is the state variable of a thermodynamic system in equilibrium. The difference in internal energy between two systems is equal to the amount of heat passed through the system minus the work done by the system. According to the first law of thermodynamics, the energy of the universe is constant. It can be transferred between the system and its environment, but it cannot be produced or destroyed. The first law of the formula of thermodynamics delta Q = delta U + delta W
Class 11 Physics Chapter 11 Multiple Choice Questions
At a given volume and temperature, the pressure of a gas
Internal energy of a perfect gas is
Thermodynamics is concerned with the
When steam is converted into water, internal energy of the system
Limitations of the first law of thermodynamics
- A limitation of the first law of thermodynamics is that it does not indicate the direction of heat flow.
- It is not possible to reverse the program. In reality, the heat is not entirely converted into work. If it is possible to convert all heat into work, we can make ships travel across oceans by extracting heat from seawater.
- Does not distinguish whether the process is spontaneous or not.
The Second Law of Thermodynamics in 11th Physics
According to the second law of thermodynamics, any spontaneous event will always result in an increase in the entropy of the universe. Basically, the law states that the entropy of an isolated system will never decrease over time. In some cases where the system is in thermodynamic equilibrium or undergoes a reversible process, the total entropy of the system and its surroundings remains constant.
The law of increasing entropy is another name for the second law. Different statements of the second law of thermodynamics is Kelvin-Planck statement. According to this it is difficult to convert all the heat emitted by a heated object into work. The working material of a heat engine absorbs heat from the hot body, converts some of it into work, and returns the rest to the cold body. No engine can convert all the heat from the source into work without wasting heat. This indicates that the receiver is required for continued operation.
Class 11 Physics Chapter 11 Topic – Carrot Engine
The Carnot engine is a theoretical thermodynamic cycle proposed by Léonard Carnot. It estimates the maximum possible efficiency of a heat engine in converting heat into work, and conversely, the maximum efficiency a heat engine can have when operating between two reservoirs. The job of a heat engine is to transfer energy from a hot area to a cold area of a space, converting some of that energy into mechanical work in the process.
The cycle can also be reversed. The system can be subject to external forces and in doing so it can transfer heat energy from a colder system to a warmer system, acting as a refrigerator or heat pump rather than a heat engine. Each thermodynamic system exists in a specific state. A thermodynamic cycle occurs when a system goes through a series of different states and eventually returns to its original state. During this cycle, the system can perform work on its environment, acting as a heat engine.
What does thermodynamics deals in Class 11 Physics Chapter 11?
In class 11, the thermodynamics is one of the important topics of Physics. It is a branch of physics, which deals with the conversion of heat into other forms of energy and vice versa. The focus is on the relationship between heat, work, temperature and energy. This branch of physics explain the transfer of energy from one place to another and from one form to another as well. The key concept of this branch is that heat is a form of energy that corresponds to a certain amount of mechanical work.
What is the significance of Zeroth law of thermodynamics in the study of 11th Physics?
The zeroth law is given in class 11 Physics chapter 11. It says that when two systems are in thermal equilibrium with a third system, these two systems are also in equilibrium with each other. When the two systems are connected by walls that allow heat to pass through, they are said to be in equilibrium. This law is considered important in the mathematical formulation of thermodynamics. Thermal equilibrium here means that the temperature of the system remains constant throughout the system.
How many laws of thermodynamics are there in chapter 11 of class 11 Physics?
In class 11 Physics, there are three laws of thermodynamics. These three laws of thermodynamics facilitate the circulation of heat and energy. The first law of thermodynamics is known as the law of conservation of energy and states that energy can neither be created nor destroyed. The energy may transferred or changed from one form to another. One way to express this law is that the change in internal energy (Q) is given by the sum of the heat (q) passing through the environment or boundary and the work done (w). Second Law says that an isolated system always increases as it progresses towards thermal equilibrium, which is the state of maximum entropy. The Third Law states that the entropy of a system approaches a constant value and the temperature reaches zero.