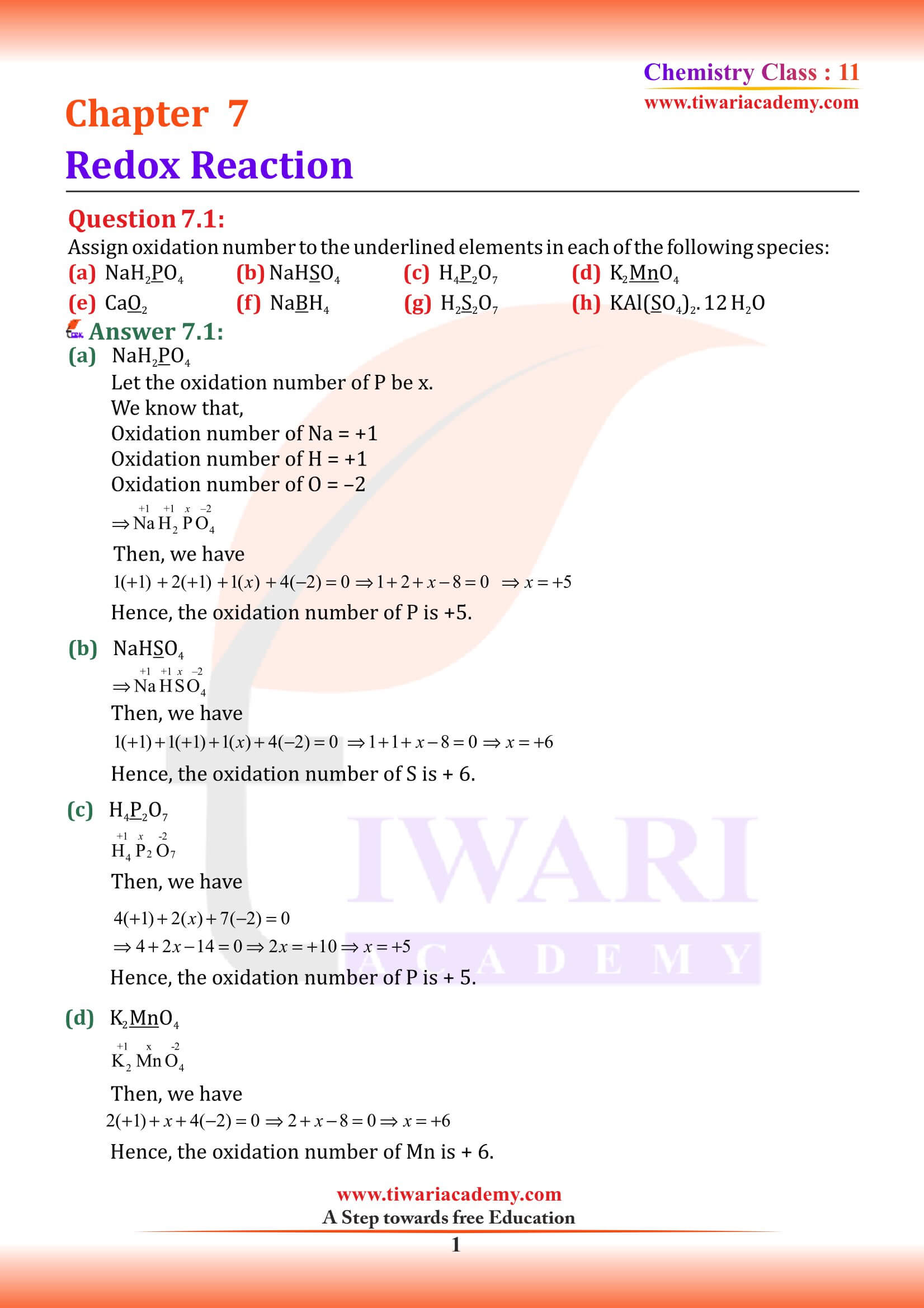
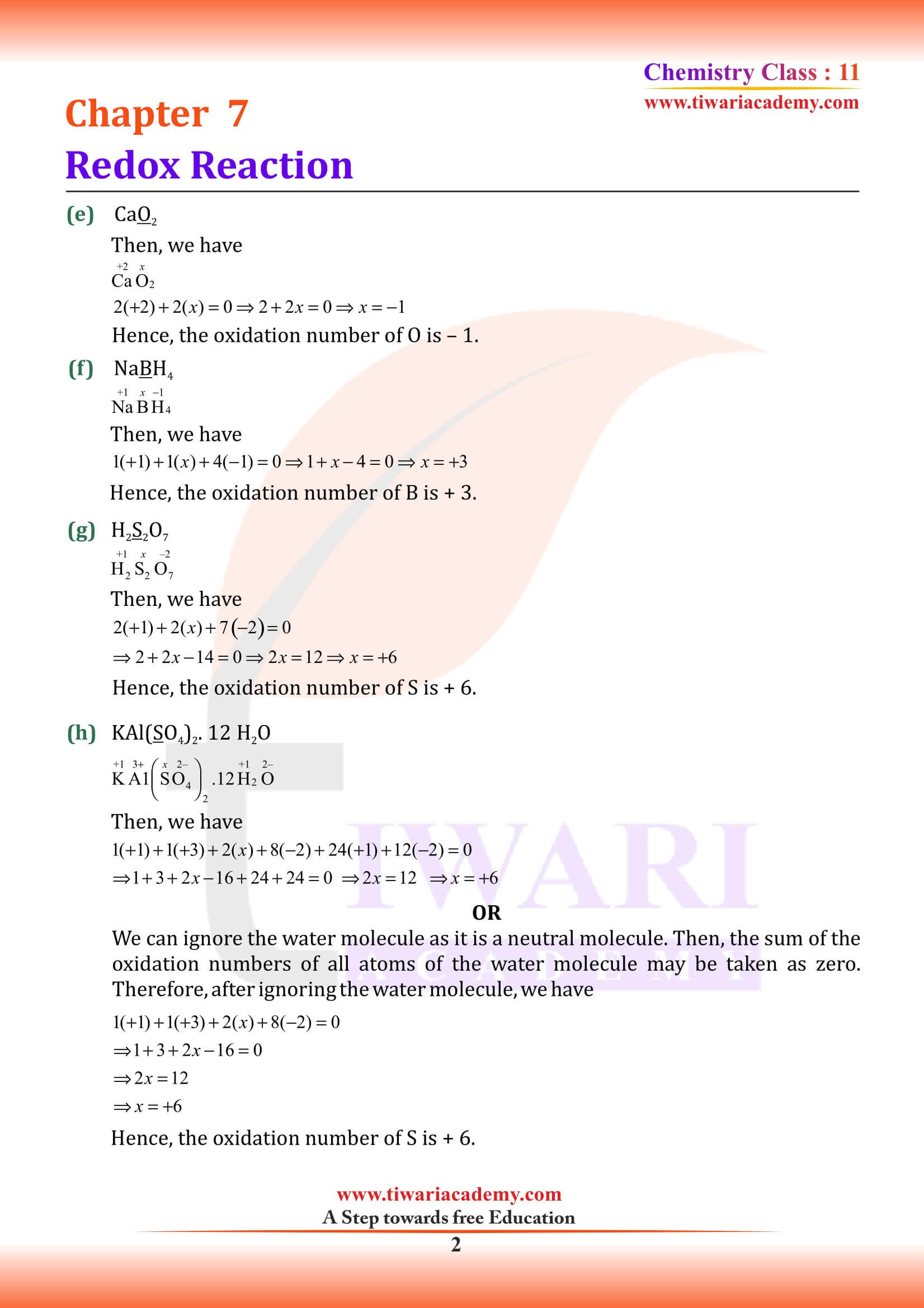
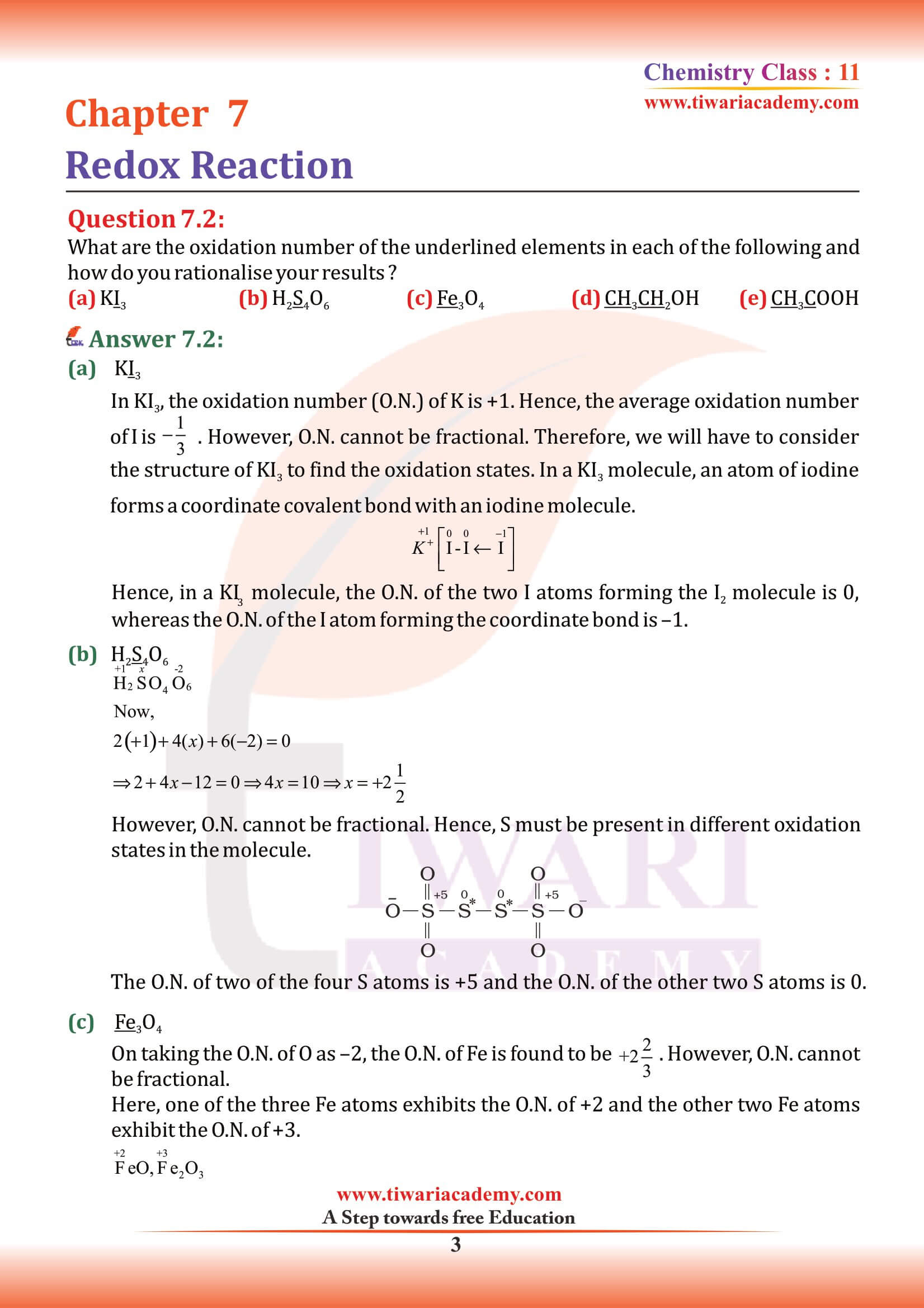
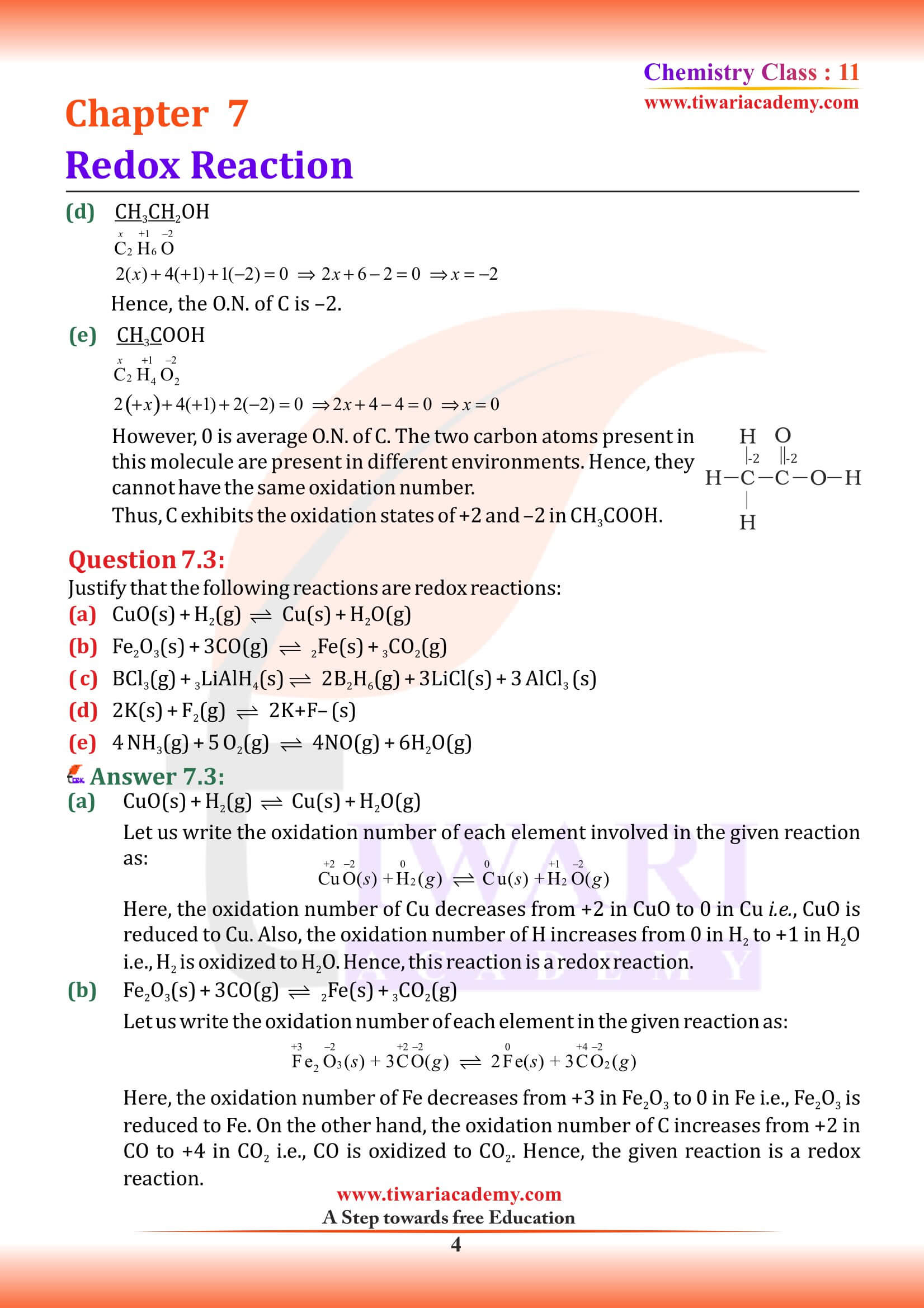
NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions in Hindi and English Medium with MCQ, Important Extra Questions, Exercises and Intext questions for CBSE session 2025-26. Get here the PDF and videos for solutions of Class 11 Chemistry Chapter 7 for the preparation of exams.
NCERT Solutions for Class 11 Chemistry Chapter 7
- Class 11 Chemistry Chapter 7 NCERT Solutions
- Class 11 Chemistry Chapter 7 NCERT Book
- Class 11 Chemistry Chapter 7 Hindi Medium
- Class 11 Chemistry Chapter 7 Revision Book
- Class 11 Chemistry Chapter 7 Study Material 1
- Class 11 Chemistry Chapter 7 Study Material 2
- Class 11 Chemistry Chapter 7 Assignment 1
- Class 11 Chemistry Chapter 7 Assignment 2
- Class 11 Chemistry Chapter 7 Assignment 3
- Class 11 Chemistry Chapter 7 Assignment 4
- Class 11 Chemistry NCERT Solutions
- Class 11 all Subjects NCERT Solutions
Redox Reactions
Redox is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. The formation of hydrogen fluoride is an example of a redox reaction.
2HgCl₂(aq) + SnCl₂(aq) ⟶ Hg₂Cl₂(s) + SnCl₄(aq)
Class 11 Chemistry Chapter 7 MCQ
Which of the following is not an example of redox reaction?
The oxidation number of an element in a compound is evaluated on the basis of certain rules. Which of the following rules is not correct in this respect?
Which of the following arrangements represent increasing oxidation number of the central atom?
The largest oxidation number exhibited by an element depends on its outer electronic configuration. With which of the following outer electronic configurations the element will exhibit largest oxidation number?
Oxidation and Reduction
Oxidation: The term oxidation is defined as the addition of oxygen/electronegative element to a substance or removal of hydrogen/ electropositive element from a substance.
Reduction: The term reduction has been broadened these days to include removal of oxygen/electronegative element from a substance or addition of hydrogen/ electropositive element to a substance.
Example:
2FeCl₃ (aq) + H₂ (g) ⟶ 2FeCl₂ (aq) + 2HCl(aq)
(Removal of electronegative element, chlorine from ferric chloride)
Class 11 Chemistry Chapter 7 Extra Important Questions
MnO₄²⁻ undergoes disproportionation reaction in acidic medium but MnO₄⁻ does not. Give reason.
In MnO₄⁻, Mn is in the highest oxidation state i.e. +7. Therefore, it does not undergo disproportionation. MnO₄²⁻ undergoes disproportionation as follows:
3MnO₄²⁻ + 4H⁺ → 2MnO₄⁻ + MnO₂ + 2H₂O
Why do these compounds differ in their reactivity?
PbO and PbO₂ react with HCl according to following chemical equations:
2PbO + 4HCl → 2PbCl₂ + 2H₂O
PbO₂ + 4HCl → PbCl₂ + Cl₂ + 2H₂O
2PbO + 4HCl → 2PbCl₂ + 2H₂O (Acid base reaction)
PbO₂ + 4HCl → PbCl₂ + Cl₂ + 2H₂O (Redox reaction)
(The oxidation number of lead in the oxides)
Nitric acid is an oxidising agent and reacts with PbO but it does not react with PbO₂. Explain why?
PbO is a basic oxide and simple acid base reaction takes place between PbO and HNO₃. On the other hand, in PbO₂ lead is in + 4 oxidation state and cannot be oxidised further. Therefore, no reaction takes place. Thus, PbO₂ is passive, only PbO reacts with HNO₃.
2PbO + 4HNO₃ → 2Pb(NO₃) ₂ + 2H₂O (Acid base reaction)
Redox Reactions in Terms of Electron Transfer Reactions
half reactions that involve loss of electrons are called oxidation reactions. Similarly, the half reactions that involve gain of electrons are called reduction reactions.
Some important terms regarding redox reactions
- Oxidation: Loss of electron(s) by any species.
- Reduction: Gain of electron(s) by any species.
- Oxidising agent: Acceptor of electron(s).
- Reducing agent: Donor of electron(s).
Oxidation Number
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set
of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative element.
Types of Redox Reactions
There are mainly three types of redox reactions are as following:
- Combination reactions
- Decomposition reactions
- Displacement reactions
Combination Reactions
Either A and B or both A and B must be in the elemental form for such a reaction to be a redox reaction. All combustion reactions, which make use of elemental dioxygen, as well as other reactions involving elements other than dioxygen, are redox reactions. Some important examples of this category are:
C(s) + O₂ (g) ⟶ CO₂ (g)
Class 11 Chemistry Chapter 7 Multiple Choice Questions
Identify disproportionation reaction
Which of the following elements does not show disproportionation tendency?
Identify the correct statement (s) in relation to the following reaction: Zn + 2HCl → ZnCl₂ + H₂
Identify the correct statements with reference to the given reaction P₄ + 3OH⁻ + 3H₂O → PH₃ + 3H₂PO₂⁻
Decomposition Reactions
Decomposition reactions are the opposite of combination reactions. Precisely, a decomposition reaction leads to the breakdown of a compound into two or more components at least one of which must be in the elemental state. Examples of this class of reactions are:
2H₂O (l) ⟶ 2H₂ (g) + O₂ (g)
Displacement Reactions
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
X + YZ ⟶ XZ + Y
Displacement reactions fit into two categories:
- (i) Metal displacement
- (ii) Non-metal displacement.