Class 10 Science Chapter 1 Notes of Chemical Reactions and Equations for the preparation of exams. 10 Science Chemistry Chapter 1 notes are in such a way that it covers the entire chapter clearing the concepts of Chemical Reactions, Equations involves and types of reactions with examples. These are prepared for the academic session 2025-26 CBSE Exams based on latest CBSE Syllabus 2025-26. Contents according to need may be added during the session, if required.
Class 10 Science Chapter 1 Notes for 2025-26
| Class: | 10 |
| Subject: | Science |
| Contents: | Notes for Revision |
Class 10 Science Chemistry Chapter 1 Notes for Exams
Class 10 Science Chapter 1 Chemical Reactions and Equations Notes of Chemistry are given below updated for new academic session 2025-26. Important Questions on Chemical Reactions and Equations and previous years questions are also available to use free. Download Apps and Solutions based on latest NCERT Books and current CBSE Syllabus 2025-26.
Notes are prepared for the quick revision of 10 Science Chapter 1 for exams. Reading NCERT Books is good habits for understanding the concepts. These notes provide only revision of the chapter. Ask your doubts in Discussion Forum and get the proper answers from the experts. Important Questions based on Chapter 1 Chemical Reactions and Equations contains all the possible questions from NCERT.
Chemical Reactions
Such a process in which breaking and making of bonds between different atoms to produces substances of new property is known as chemical reaction. We should have to determine the chemical reaction has taken place, if there is:
Change in state.
Change in colour.
Evolution of a gas.
Change in temperature.
Class 10 Science Chapter 1 Extra Questions
What is a Chemical Equation?
The representation of chemical reaction in the form of formulae of reactants and products separated by an arrow marks is known as a Chemical Equation.
What are different type of Chemical Reactions?
The types of reaction are as follow;
(1) Combination reaction
(2) Decomposition reaction
(3) Displacement reaction
(4) Double displacement reaction
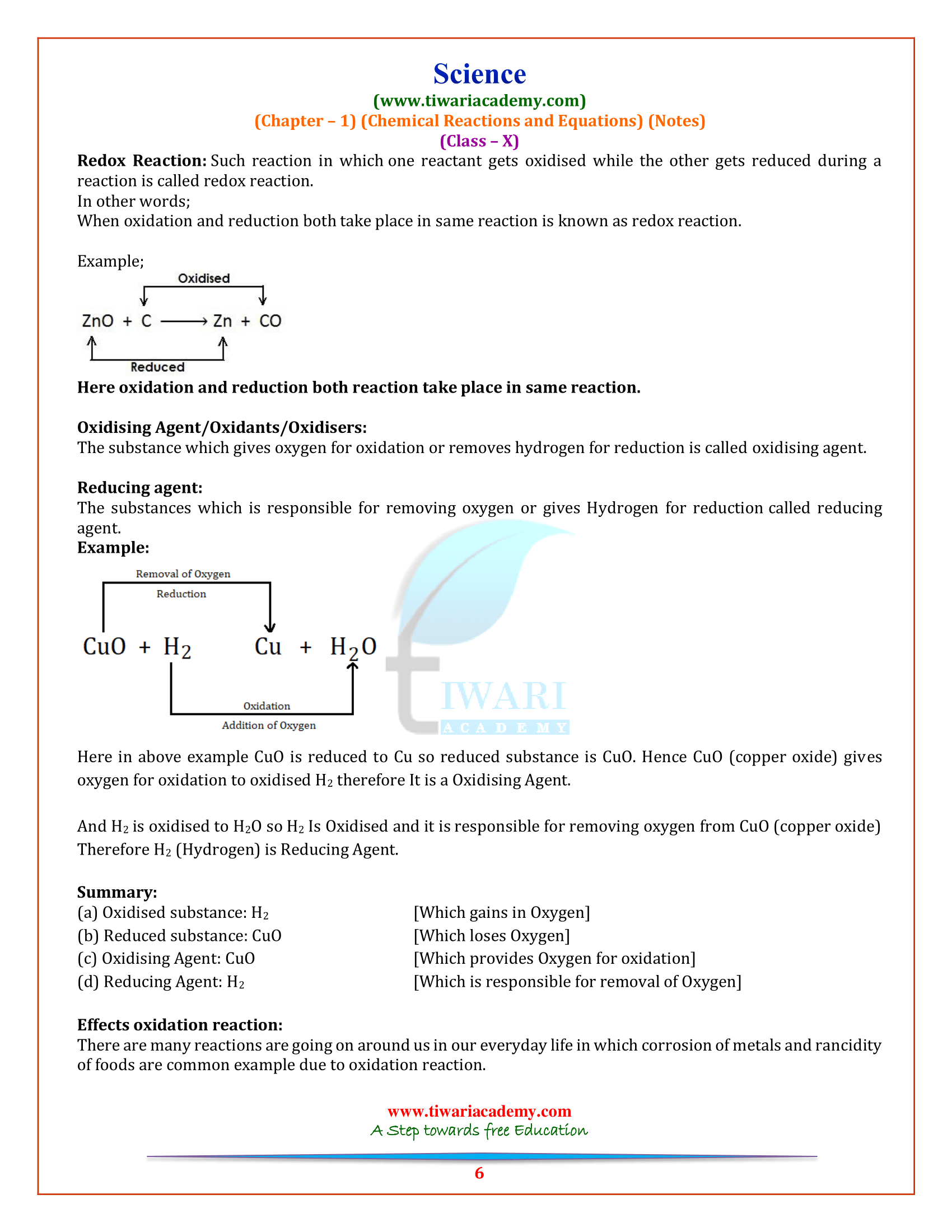
(5) Oxidation and reduction reaction
What is Decomposition Reaction?
The reaction in which a single reactant breaks down into two or more than two simpler products is known as decomposition reaction.
General form of Decomposition Reaction is given by:
A → B + C
What are Exothermic Reactions?
Reactions in which heat is released along with the formation of products are called exothermic.
During the reaction large amount of heat (energy) is evolved and this heat makes the reaction mixture warm.
Respiration is an Exothermic Reaction
When we take food to get energy to stay alive. During digestion, food is broken down into simpler substances likes carbohydrates and other nutrients. These carbohydrates further broken down to form glucose. This glucose combines with oxygen in cells of our body in the process of cellular respiration and provide energy. Therefore, respiration is also an exothermic reaction.
Corrosion
The process in which metals looses their surface gradually by the action of air, water and moisture is called corrosion.
In other words:
The process in which metals surface get corroded by reaction with air, water and moisture is called corrosion.
Corrosion is not a rusting while rusting causes corrosion.
Preventing Corrosion
There are following method to prevent corrosion:
(i) By galvanization
(ii) By painting metal surfaces
(iii) By oiling or greasing metal surfaces
Download NCERT Books and Offline Apps based on new CBSE Syllabus 2025-26. Ask your doubts related to NIOS or CBSE Board and share your knowledge with your friends and other users through Discussion Forum.